Avogadro's Number In Standard Form
Avogadro's Number In Standard Form - Web the number of units in one mole of any substance is called avogadro’s number or avogadro’s constant. It's the basis for the mole unit of measurement, which provides an easy. It is equal to 602,252,000,000,000,000,000,000, or in. For example it is also pretty. Web avogadro's number ävōgä´drō [for amedeo avogadro ], number of particles contained in one mole of any substance; Web the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass of a substance. Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). Web avogadro's number is the number of particles in one mole of anything. The number 6.022 × 10²³ is known as avogadro's number or. Web the resultant avogadro's number equality is shown below.
This number represents one mole of atoms, molecules, or ions. It's easy to find the mass of. In this context, it is the number of atoms in one mole of an element. Web avogadro's number is used in chemistry when you need to work with very large numbers. It is equal to 6.022140857×10 23. It's the basis for the mole unit of measurement, which provides an easy. The units may be electrons,. Web the number of units in one mole of any substance is called avogadro’s number or avogadro’s constant. Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). Web the resultant avogadro's number equality is shown below.
Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). Web avogadro's number is used in chemistry when you need to work with very large numbers. Web the most accurate statement with regard to the significance of avogadro's number, 6.02×10 23. Web for example, later on in this course we will be working with avogadro’s number (the number of elementary entities in 1 mole of a substance). Web the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass of a substance. The number 6.022 × 10²³ is known as avogadro's number or. The avogadro’s number full is equal to 6.02214076 × 10 23. 1 mol si = 6.02 × 1023 si atoms. This number represents one mole of atoms, molecules, or ions. Because the given chemical name is an elemental name, the indicator word .
Avogadros Stoichiometry YouTube
Web the most accurate statement with regard to the significance of avogadro's number, 6.02×10 23. Web the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass of a substance. It is equal to 6.022140857×10 23. Web the number of units in one mole of.
How many moles of hydrogen are in 100 L of hydrogen at STP? 100 L = mol
To calculate the molecular mass of a covalent compound and the formula mass of an ionic compound. This number represents one mole of atoms, molecules, or ions. Web avogadro's number is used in chemistry when you need to work with very large numbers. The number 6.022 × 10²³ is known as avogadro's number or. It's easy to find the mass.
Avogadro's Law Definition, Formula, Equation and Examples
Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). It's easy to find the mass of. Web in fact about 602200000000000000000000 of them. It is a number, just as is dozen, and thus is dimensionless. Web avogadro's number is the number of particles in one mole of.
PPT Avogadro’s Number PowerPoint Presentation, free download ID4492286
Web the most accurate statement with regard to the significance of avogadro's number, 6.02×10 23. 1 mol si = 6.02 × 1023 si atoms. Web things to understand about avogadro's number. It is a huge number, far greater in magnitude than. Web the mole is a unit used to measure the number of atoms, molecules, or (in the case of.
What Is Avogadro's Number? Definition and Importance
The units may be electrons,. Web the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass of a substance. Avogadro's number and the mole. It's easy to find the mass of. Web the number of units in one mole of any substance is called.
Using Avogadro's Number YouTube
Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). The avogadro’s number full is equal to 6.02214076 × 10 23. Web in fact about 602200000000000000000000 of them. It is the number of atoms in exactly 12 grams of. In this context, it is the number of atoms.
Using Avogadro's Number YouTube
Web avogadro's number is the number of particles in one mole of anything. Web the mole is a unit used to measure the number of atoms, molecules, or (in the case of ionic compounds) formula units in a given mass of a substance. For example it is also pretty. The units may be electrons,. It's easy to find the mass.
Mole, avogadro's number and calculations based on balanced chemical e…
1 mol si = 6.02 × 1023 si atoms. It is a huge number, far greater in magnitude than. Web one mole of a substance is equal to 6.022 × 10²³ units of that substance (such as atoms, molecules, or ions). Web avogadro's number is used in chemistry when you need to work with very large numbers. The number 6.022.
Difference Between Avogadro’s Constant and Avogadro’s Number
It is the number of atoms in exactly 12 grams of. The avogadro’s number full is equal to 6.02214076 × 10 23. Avogadro's number and the mole. Web avogadro's number is used in chemistry when you need to work with very large numbers. It's the basis for the mole unit of measurement, which provides an easy.
What is Avogadro's number? Trivia Questions
For example it is also pretty. In this context, it is the number of atoms in one mole of an element. The number 6.022 × 10²³ is known as avogadro's number or. Because the given chemical name is an elemental name, the indicator word . 1 mol si = 6.02 × 1023 si atoms.
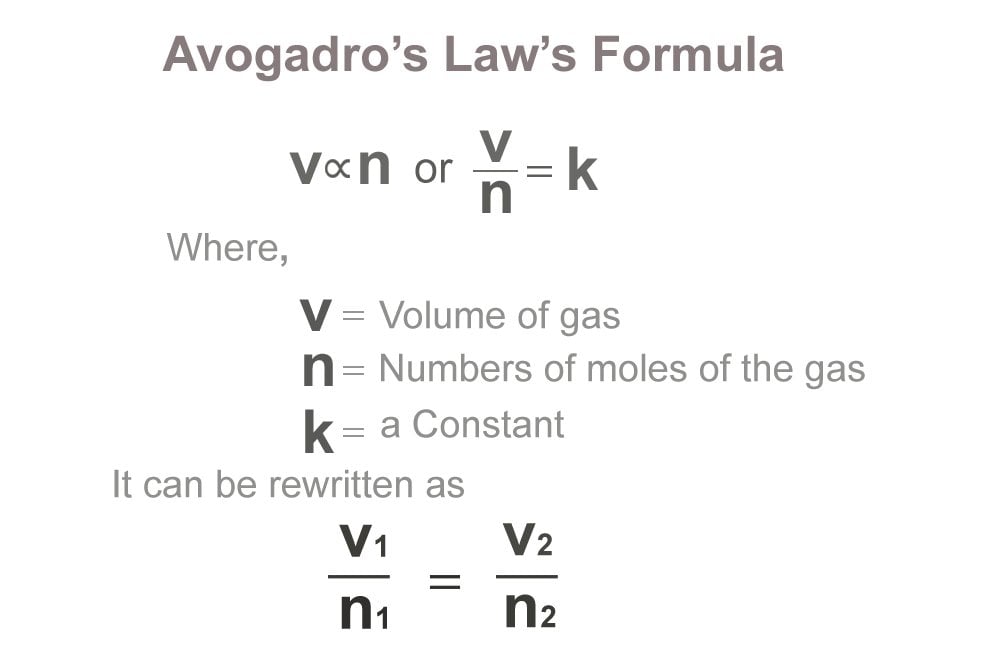
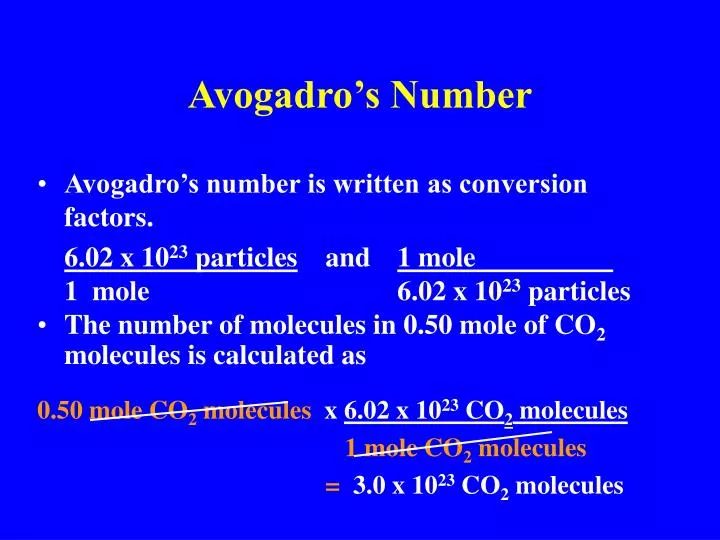
Web One Mole Of A Substance Is Equal To 6.022 × 10²³ Units Of That Substance (Such As Atoms, Molecules, Or Ions).
It is equal to 6.022140857×10 23. Web things to understand about avogadro's number. To calculate the molecular mass of a covalent compound and the formula mass of an ionic compound. Web avogadro's number is the number of particles in one mole of anything.
It Is A Number, Just As Is Dozen, And Thus Is Dimensionless.
It is equal to 602,252,000,000,000,000,000,000, or in. The number 6.022 × 10²³ is known as avogadro's number or. Furthermore, avogadro’s number refers to the number of particles that exist in one mole of any. Web for example, later on in this course we will be working with avogadro’s number (the number of elementary entities in 1 mole of a substance).
In This Context, It Is The Number Of Atoms In One Mole Of An Element.
This number represents one mole of atoms, molecules, or ions. The avogadro’s number full is equal to 6.02214076 × 10 23. Web in fact about 602200000000000000000000 of them. Web avogadro's number, or avogadro's constant, is the number of particles found in one mole of a substance.
It Is The Number Of Atoms In Exactly 12 Grams Of.
Web the resultant avogadro's number equality is shown below. Because the given chemical name is an elemental name, the indicator word . It's easy to find the mass of. Web the most accurate statement with regard to the significance of avogadro's number, 6.02×10 23.

.PNG)