Chapter 16 Thermal Energy And Heat
Chapter 16 Thermal Energy And Heat - Web summary 16.1 thermal energy and matter heat flows spontaneously from hot objects to cold objects. Borders edited by lenny leta. Learn about how thermal energy is used or can be used in many different situations,. Heat flows spontaneously (without assistance) from hot objects to cold objects. Depends on mass, temperature, and phase of an object. A measure of how hot. The two main types of heat engines are the _____ and the _____ combustion. Particles spread out & the substance expands what happens as thermal energy. Circulation of a fluid in a loop as the fluid alternately heats up and cools down. The transfer of thermal energy from one body of matter to another because of a temperature difference.
Heat flows spontaneously (without assistance) from hot objects to cold objects. Enter all required information in the necessary fillable areas. Borders edited by lenny leta. Web thermodynamics is the study of conversions betwe= en _____ energy and other forms of energy. Web the transfer of thermal energy from one object to another because of a difference in temperature. Web name two variables that affect the thermal energy of an object. Learn about how thermal energy is used or can be used in many different situations,. The process occurred in water so that the metal would not melt due to the heat. What principle explains how a calorimeter is used to measure the specific heat. Count rumford rumford studied the process of drilling holes in the barrels of cannons.
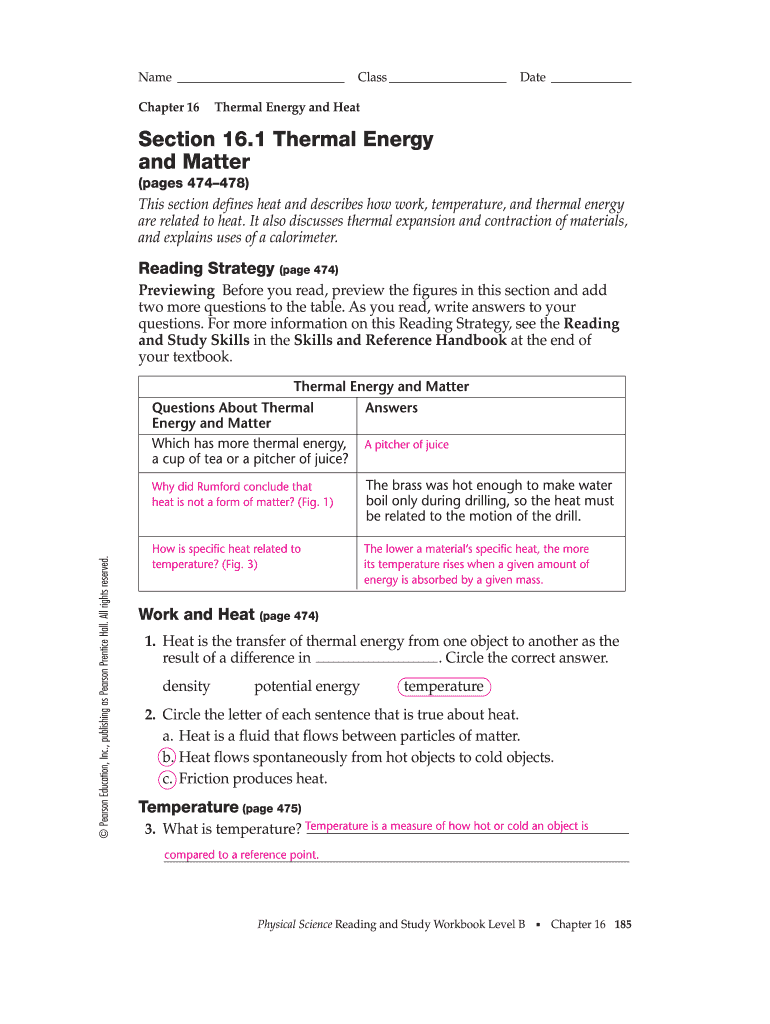
Heat is written with the symbol q or q, and it has units of joules ( \text j j ). How do the temperature increases of different materials depend on their specific heats? Learn about how thermal energy is used or can be used in many different situations,. Chapter 16 thermal energy and heat. Web circle the letter of each sentence that is true about heat. A measure of how hot or cold an object is compared to a reference point. An increase in volume of a material due to a temperature increase. Heat is the transfer of thermal energy from one object to another because of a temperature difference. In the 1700’s most scientists thought that heat was a fluid called caloric that. What principle explains how a calorimeter is used to measure the specific heat.
Thermal Energy & Heat Transfer Middle School Science Unit Teaching
Click the card to flip 👆 definition 1 / 17 celsius kelvin fahrenheit click the card to flip 👆 flashcards learn. According to the second law of thermodynamics, w= hat must happen for thermal energy. Count rumford rumford studied the process of drilling holes in the barrels of cannons. Enter all required information in the necessary fillable areas. The transfer.
Difference Between Heat and Thermal Energy Difference Between
Borders edited by lenny leta. How do the temperature increases of different materials depend on their specific heats? Web circle the letter of each sentence that is true about heat. The amount of heat needed to raise the temperature of one gram of a material by one degree celsius. A measure of how hot.
PPT Chapter 16 Thermal Energy and Heat PowerPoint Presentation, free
Depends on mass, temperature, and phase of an object. How do the temperature increases of different materials depend on their specific heats? Web summary 16.1 thermal energy and matter heat flows spontaneously from hot objects to cold objects. Heat is the transfer of thermal energy from one object to another because of a temperature difference. Web the transfer of thermal.
Section 16 1 Thermal Energy And Matter Fill Online, Printable
Web physical science chapter 16 term 1 / 17 which of the following is a unit of temperature? Web scientists define heat as thermal energy transferred between two systems at different temperatures that come in contact. D.the transfer of thermal energy from one object to another is heat… Instrument used to measure thermal energy. Borders edited by lenny leta.
PPT Chapter 16 Thermal Energy and Heat PowerPoint Presentation
What principle explains how a calorimeter is used to measure the specific heat. Web name two variables that affect the thermal energy of an object. Depends on mass, temperature, and phase of an object. Count rumford rumford studied the process of drilling holes in the barrels of cannons. The amount of heat needed to raise the temperature of one gram.
️Thermodynamics Worksheet Answer Key Free Download Gambr.co
In the 1700’s most scientists thought that heat was a fluid called caloric that. Depends on mass, temperature, and phase of an object. Web the transfer of thermal energy from one object to another because of a difference in temperature. An increase in volume of a material due to a temperature increase. What causes thermal expansion of an object when.
How should governments subsidize cleanenergy heating?
Learn about how thermal energy is used or can be used in many different situations,. Enter all required information in the necessary fillable areas. Web adhere to our simple steps to have your chapter 16 thermal energy and heat wordwise prepared quickly: The amount of heat needed to raise the temperature of one gram of a material by one degree.
Chapter 16 Thermal Energy and Heat
Instrument used to measure thermal energy. Heat flows spontaneously (without assistance) from hot objects to cold objects. What causes thermal expansion of an object when it is heated? The transfer of thermal energy from one body of matter to another because of a temperature difference. Thermal energy is transferred without transfer of matter.
PPT Chapter 16 Thermal Energy and Heat PowerPoint Presentation, free
Matter is transferred great distances during conduction. I can describe the differences in transferring heat by conduction, convection,. This motion can generate heat. The two main types of heat engines are the _____ and the _____ combustion. What causes thermal expansion of an object when it is heated?
PPT Chapter 16 PowerPoint Presentation, free download ID6516821
Heat is written with the symbol q or q, and it has units of joules ( \text j j ). Web name two variables that affect the thermal energy of an object. A measure of how hot or cold an object is compared to a reference point. Web adhere to our simple steps to have your chapter 16 thermal energy.
Web Physical Science Chapter 16 Term 1 / 17 Which Of The Following Is A Unit Of Temperature?
Conduction can occur between materials that are not touching. A measure of how hot. The amount of heat needed to raise the temperature of one gram of a material by one degree celsius. Chapter 16 thermal energy and heat.
Web Thermal Energy Is Related To The Movement Of Particles In An Object;
Temperature is related to the average kinetic energy. What principle explains how a calorimeter is used to measure the specific heat. The transfer of energy by waves moving through space. Particles spread out & the substance expands what happens as thermal energy.
What Causes Thermal Expansion Of An Object When It Is Heated?
The amount of heat needed to raise the temperature of one gram of a material by one degree celsius. Web name two variables that affect the thermal energy of an object. A measure of how hot or cold an object is compared to a reference point. The process occurred in water so that the metal would not melt due to the heat.
Depends On Mass, Temperature, And Phase Of An Object.
Borders edited by lenny leta. Click the card to flip 👆 definition 1 / 17 celsius kelvin fahrenheit click the card to flip 👆 flashcards learn. Heat is the transfer of thermal energy from one object to another because of a temperature difference. Heat is written with the symbol q or q, and it has units of joules ( \text j j ).