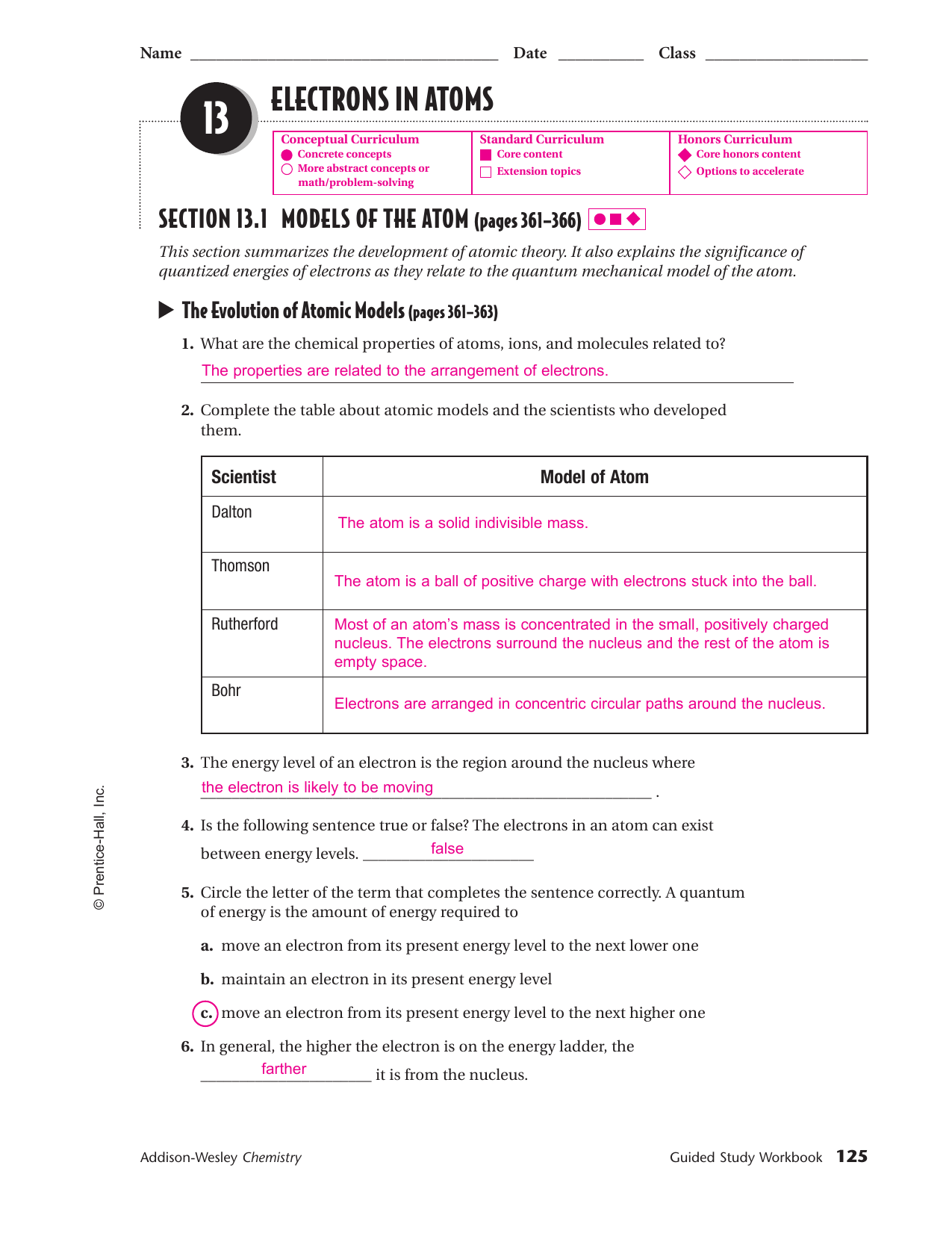
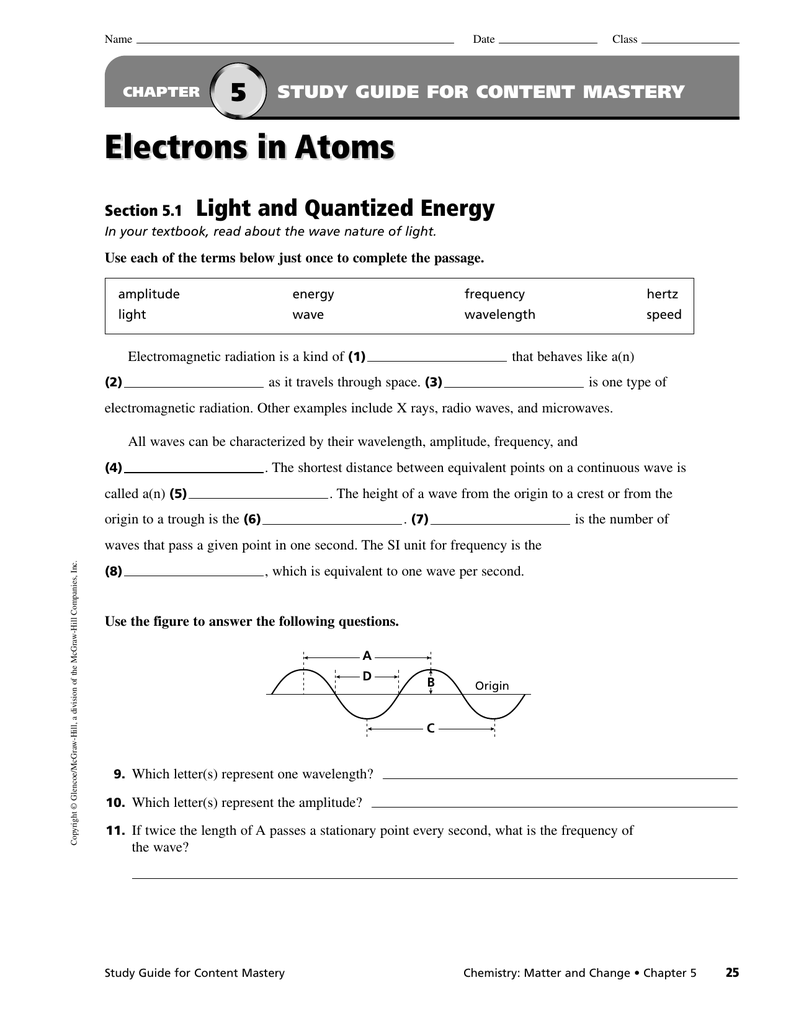
Chapter 5 Electrons In Atoms
Chapter 5 Electrons In Atoms - Arrangement of electrons around atomic nucleus. We also explain how knowing the arrangement of electrons in. Electrons exist when they're not excited. Terms in this set (18) energy levels. Electrons can jump from one level to another when excited (from the ground state to an excited state). The maximum number of electrons that. Web in this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to their energies. Tendency of electrons to enter orbitals of lowest energy first. The arrangement of electrons in a atom's ___. Electrons in an atom tend to assume the arrangement that gives the atom the ___ possible energy.
Each of these orbits has a specific number of electrons. When the electrons return to the ground state. This arrangement of electrons is the most ___ arrangement and is the atoms. Electrons in an atom tend to assume the arrangement that gives the atom the ___ possible energy. It also explains the significance of quantized energies of electrons. Objectives compare the wave and particle natures of light. Web each shell can hold two electrons which allow orbital p to hold six electrons, orbital d can hold ten electrons, and orbital f can hold fourteen electrons. Web what are the electrons doing and how do they move ? 1s1, 2s2, 2p2, 3s2, 3p3, 4s2. Web chapter5 what you’ll learn you will compare the wave and particle models of light.
Define a quantum of energy, and. The arrangement of electrons in a atom's ___. The rule that electrons occupy the orbitals of lowest energy first. You will describe how the frequency of light emitted by an atom is a unique characteristic of that atom. Is concerned with the probability of finding an electron in a certain position. Web each shell can hold two electrons which allow orbital p to hold six electrons, orbital d can hold ten electrons, and orbital f can hold fourteen electrons. Includes all forms of electromagnetic radiation which vary in their frequencies and wavelength. 4 answer by gaining or losing certain amounts of energy, electrons can make quantum jumps. 1s1, 2s2, 2p2, 3s2, 3p3, 4s2. We also explain how knowing the arrangement of electrons in.
Chapter 5 electrons in atoms
Web electrons exist in fixed pathways (orbits) around the nucleus. Objectives compare the wave and particle natures of light. Web 136 chapter 5 • electrons in atoms section 55.1.1 figure 5.1 different elements can have similar reactions with water. Electrons exist when they're not excited. Click the card to flip 👆 definition 1 / 51 ground state:
Chemistry Chp 5 Electrons In Atoms Powerpoint
Is concerned with the probability of finding an electron in a certain position. The rule that electrons occupy the orbitals of lowest energy first. Arrangement of electrons around atomic nucleus. Each of these orbits has a specific number of electrons. The maximum number of electrons that.
Electrons in Atoms
Electrons exist when they're not excited. Web the arrangement of electrons of an atom in its ground state into various orbitals around the nuclei of atoms. It also explains the significance of quantized energies of electrons. Web what are the electrons doing and how do they move ? Valence electrons are the electrons in the highest occupied principal energy level.
Chapter 5 Electrons In Atoms Answer Key THE PREMIER FEMALE DJ OF LOS
Diagrams that show valence electrons as dots. Web chapter5 what you’ll learn you will compare the wave and particle models of light. The maximum number of electrons that. Tendency of electrons to enter orbitals of lowest energy first. Arrangement of electrons around atomic nucleus.
Chapter 5 electrons in atoms
Web chapter5 what you’ll learn you will compare the wave and particle models of light. The maximum number of electrons that. Includes all forms of electromagnetic radiation which vary in their frequencies and wavelength. 1s1, 2s2, 2p2, 3s2, 3p3, 4s2. Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of.
10+ Chapter 5 Electrons In Atoms Answer Key WilliamMiran
Diagrams that show valence electrons as dots. 4 answer by gaining or losing certain amounts of energy, electrons can make quantum jumps. The rule that electrons occupy the orbitals of lowest energy first. Tendency of electrons to enter orbitals of lowest energy first. The energy levels contained within a principle energy level.
Chapter 5 electrons in atoms
This arrangement of electrons is the most ___ arrangement and is the atoms. 4 answer by gaining or losing certain amounts of energy, electrons can make quantum jumps. Chapter 5 “electrons in atoms” section 5.1 models of the atom objectives: In the second period elements, the two electrons in the 1s sublevel are called. Click the card to flip 👆.
Chapter 5 Electrons in Atoms
We also explain how knowing the arrangement of electrons in. Objectives compare the wave and particle natures of light. Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of an electron? Includes all forms of electromagnetic radiation which vary in their frequencies and wavelength. You will describe how the frequency.
Chapter 5 Electrons in Atoms
The arrangement of electrons in a atom's ___. The ratio of the number of observations in a statistical. Web 136 chapter 5 • electrons in atoms section 55.1.1 figure 5.1 different elements can have similar reactions with water. Web chemistry chapter 5 electrons in atoms term 1 / 51 difference between ground state and the excited state of an electron?.
Chemistry Chapter 5 Electrons In Atoms Study Guide Answers Study Poster
Electrons can jump from one level to another when excited (from the ground state to an excited state). • identify the inadequacies in the rutherford atomic model. You will describe how the frequency of light emitted by an atom is a unique characteristic of that atom. The arrangement of electrons in a atom's ___. It also explains the significance of.
You Will Describe How The Frequency Of Light Emitted By An Atom Is A Unique Characteristic Of That Atom.
In the second period elements, the two electrons in the 1s sublevel are called. 4 answer by gaining or losing certain amounts of energy, electrons can make quantum jumps. The maximum number of electrons that. Web chapter5 what you’ll learn you will compare the wave and particle models of light.
Electrons Can Jump From One Level To Another When Excited (From The Ground State To An Excited State).
Click the card to flip 👆 definition 1 / 51 ground state: Web the height of a wave from the origin to a crest, or from the origin to the trough. The ratio of the number of observations in a statistical. Terms in this set (18) energy levels.
• Identify The Inadequacies In The Rutherford Atomic Model.
Web the arrangement of electrons of an atom in its ground state into various orbitals around the nuclei of atoms. The rule that electrons occupy the orbitals of lowest energy first. Web each shell can hold two electrons which allow orbital p to hold six electrons, orbital d can hold ten electrons, and orbital f can hold fourteen electrons. Define a quantum of energy, and.
Objectives Compare The Wave And Particle Natures Of Light.
Web electrons exist in fixed pathways (orbits) around the nucleus. Is concerned with the probability of finding an electron in a certain position. Electrons in an atom tend to assume the arrangement that gives the atom the ___ possible energy. Web 136 chapter 5 • electrons in atoms section 55.1.1 figure 5.1 different elements can have similar reactions with water.