Chapter 7 Review Chemical Formulas And Chemical Compounds
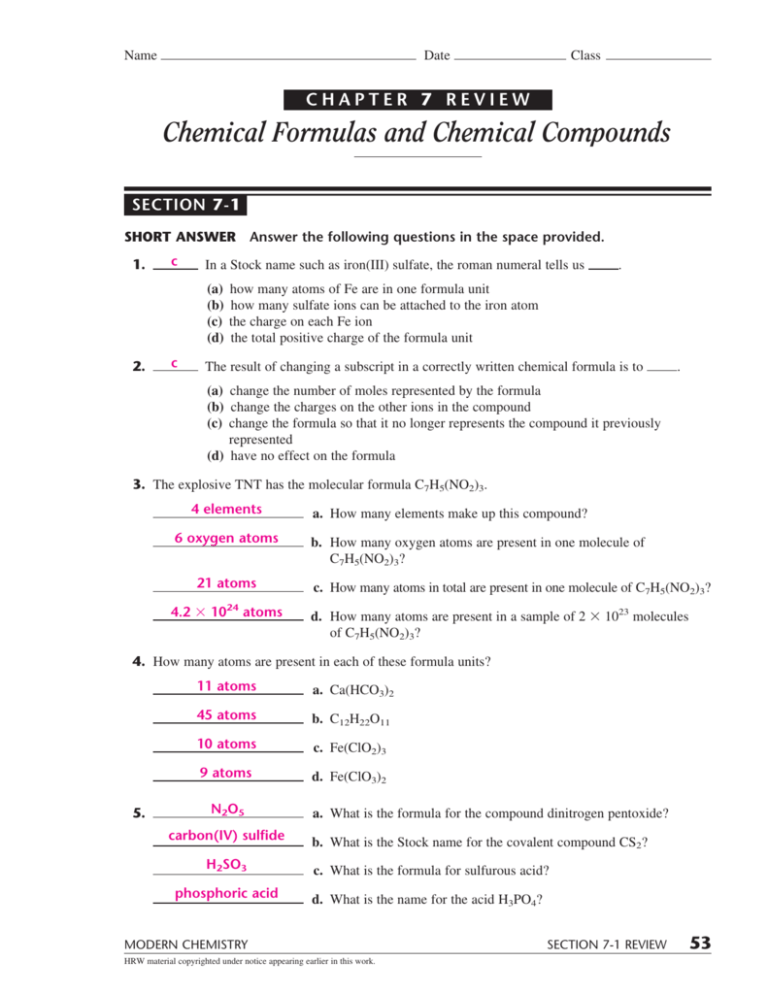
Chapter 7 Review Chemical Formulas And Chemical Compounds - Chemical formula writing worksheet review with answer key.pdf. Number of atoms of each element in one molecule of a compound c. Chapter 5 the legislative branch 41 terms. Some of the group 2 metals react with oxygen to form peroxides. Significance of a chemical formula a. _____ changing a subscript in a correctly written chemical formula A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. Click the card to flip 👆. 6= ethane (2 carbon atoms, 6 hydrogen atoms) b. (c) the charge on each fe ion.
Some of the group 2 metals react with oxygen to form oxides. The number of moles of the compound b. The oxidation numbers of all the elements in the compound d. Total number of positive and negative charges must be equal. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. Click the card to flip 👆. What is true about the charges in a binary compound. Number of atoms of each element in one molecule of a compound c. C in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. A chemical formula for a molecular compound represents the composition of.
(b) how many sulfate ions can be attached to the iron atom. The oxidation numbers of all the elements in the compound d. A chemical formula for a molecular compound represents the composition of. Write formulas for the following compounds… Chemical compounds and chemical formulas. (b) how many sulfate ions can be attached to the iron atom. In a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula. The formula mass of the compound c. Chapter 5 the legislative branch 41 terms. Click the card to flip 👆.
Chemical Compounds 1er Edition 1. 3 Volume in pdf Science
Chemical compounds and chemical formulas. (b) how many sulfate ions can be attached to the iron atom. Chapter 5 the legislative branch 41 terms. (d) the total positive charge of the formula. 6= ethane (2 carbon atoms, 6 hydrogen atoms) b.
Chapter 7 Chemical Formulas and Chemical Compounds
Atomic mass of each element. (b) how many sulfate ions can be attached to the iron atom. Total number of positive and negative charges must be equal. Number of atoms of each element in one molecule of a compound c. Click the card to flip 👆.
PPT Modern Chemistry Chapter 7 Chemical Formulas & Chemical Compounds
Chemical compounds and chemical formulas. What is the empirical formula for a compound. A chemical formula for a molecular compound represents the composition of. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. What is true about the charges in a binary compound.
Chemical Compounds, their Common Names, Formulas & Uses Download PDF
C) type of bond holding particles together in a compound. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space provided. The atoms in a pure. Chemical formulas and chemical compounds (mixed review) copper (ii) carbonate click the card to flip 👆 cuco₃ click the.
10+ Chapter 7 Review Chemical Formulas And Chemical Compounds Answer
The empirical formula for a compound shows the symbols of the elements with subscripts indicating. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. Naming ionic and covalent compounds. Chemical compounds and chemical formulas. Oxidation numbers are assigned to the atoms composing a compound or ion in order to indicate the general distribution.
PPT Chapter 7 Chemical Formulas & Compounds PowerPoint Presentation
Write the formulas for these compounds. The number of moles of the compound b. What is true about the charges in a binary compound. Number of atoms or ions of each element that are combined in the compound. Web answer the following questions in the space provided.
CHEMISTRY at Crossroads Middle School StudyBlue
(b) how many sulfate ions can be attached to the iron atom. Number of atoms or ions of each element that are combined in the compound. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. _____ changing a subscript in a correctly written.
Compounds Chemical Formulae Teaching Resources
Naming ionic and covalent compounds. 6= ethane (2 carbon atoms, 6 hydrogen atoms) b. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. Web chapter 7 review : Other sets by this creator.
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
Web chapter 7 review : Click the card to flip 👆 a chemical formula includes the symbols of the elements in the compound. Web a) charge on an ion. Click the card to flip 👆. D) distribution of electrons among the bonded particles in a compound.
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
Number of atoms or ions of each element that are combined in the compound. The oxidation numbers of all the elements in the compound d. (d) the total positive charge of the formula unit. What is true about the charges in a binary compound. Web benzene, chemical compound, carbonyl compounds, carboxylic acids, acyl compounds, chemical bonding, chemistry of life, electrode.
Naming System Or Binary Ionic Compounds Involves Combining Names Of Compounds.
C in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. Covalent compounds naming and review. Chapter 5 the legislative branch 41 terms. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate.
Chemical Formula Writing Worksheet Review With Answer Key.pdf.
Write the formulas for these compounds… Web a) charge on an ion. Click the card to flip 👆. Atomic mass of each element.
Formula Mass, Molar Mass, And Percentage Composition Can Be Calculated.
Other sets by this creator. Web chemistry chapter 7 review (names and formulas) term. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space provided. Oxidation numbers are assigned to the atoms composing a compound or ion in order to indicate the general distribution of electrons among the bonded atoms in the compound or ion.
What Is The Empirical Formula For A Compound.
The oxidation numbers of all the elements in the compound d. Web chapter 7 review : Web review the common reactions of group 2 metals in the elements handbook (appendix a), and answer the following questions: What is true about the charges in a binary compound.