Form Fda 483 Inspectional Observations
Form Fda 483 Inspectional Observations - Web this fda form 483 document includes the inspector’s observations and judgment regarding the conditions that may constitute violations of the food drug and. An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any. They are inspectional observations, and do not represent a final. They are inspectional observations, and do not represent a final agency. You provided a response to the. There may be other objectionable. Discover how a leading companies uses our data to always be prepared for inspections Web an fda 483 observation, or “inspectional observation,” is a notice sent by the fda to highlight any potential regulatory violations found during a routine inspection. So it’s an official closing of the inspection. Web the form 483 officially known as “notice of inspectional observations4” sometimes, along with the form 483 fda also issues establishment inspection report (eir) it specifies.
Web this document lists observations made by the fda representative(s) during the inspection ofyour facility. Web form fda 483, [2] inspectional observations, is a form used by the fda to document and communicate concerns discovered during these inspections. Web this document lists observations made by 1he fda representative(s) during the inspection of your facility. Web an fda 483 observation, or “inspectional observation,” is a notice sent by the fda to highlight any potential regulatory violations found during a routine inspection. Specifically, the firm has not. Web fda form 483 after each inspection, fda prepares a written list of discrepancies noted during the inspection. Web the fda form 483 is a report which does not include observations of questionable or unknown significance at the time of the inspection. They are inspectional observations, and do not represent a final agency. The list is known as form 483 or notice of inspectional. Once it’s given to you, they have to.
Discover how a leading companies uses our data to always be prepared for inspections Web this document lists observations made by the fda represcntative(s) during the inspection ofyour facility. An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any. Web this fda form 483 document includes the inspector’s observations and judgment regarding the conditions that may constitute violations of the food drug and. Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. Web an fda form 483 observation, also known as an inspectional observation, is a notice sent by the fda to inform an organization of potential facility,. Web what are fda form 483 observations? Specifically, the firm has not. Web i!observations</strong> made by the. Once it’s given to you, they have to.
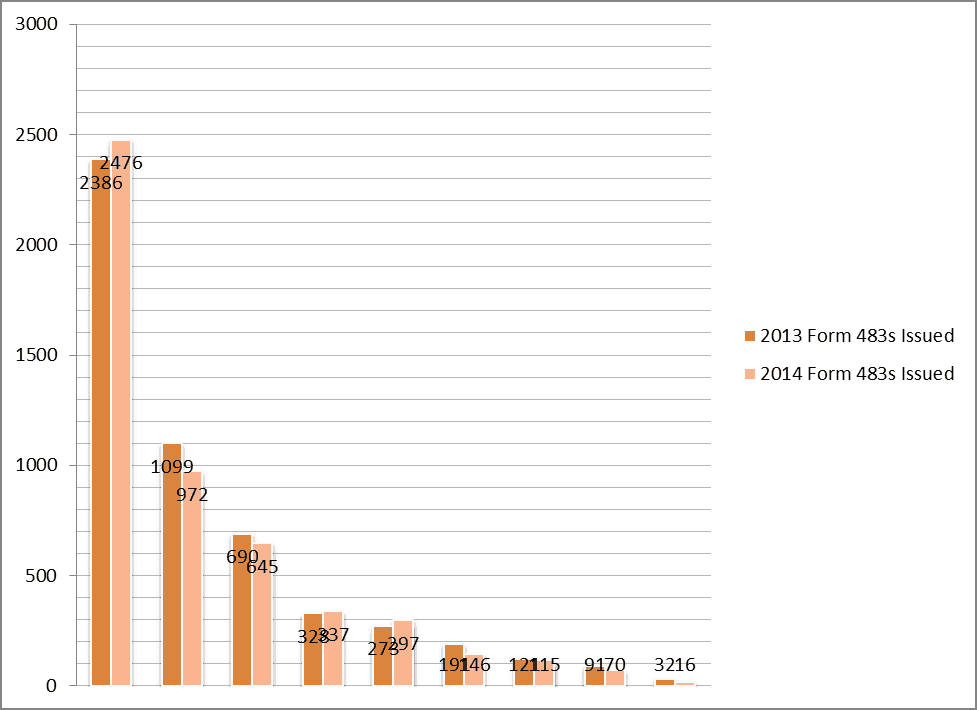
FDA Form 483 (Inspectional Observations) Top Violations 2013
Web this document lists observations made by the fda representative(s) during the inspection of your facility. Web fda form 483 after each inspection, fda prepares a written list of discrepancies noted during the inspection. You provided a response to the. The list is known as form 483 or notice of inspectional. Specifically, the firm has not.
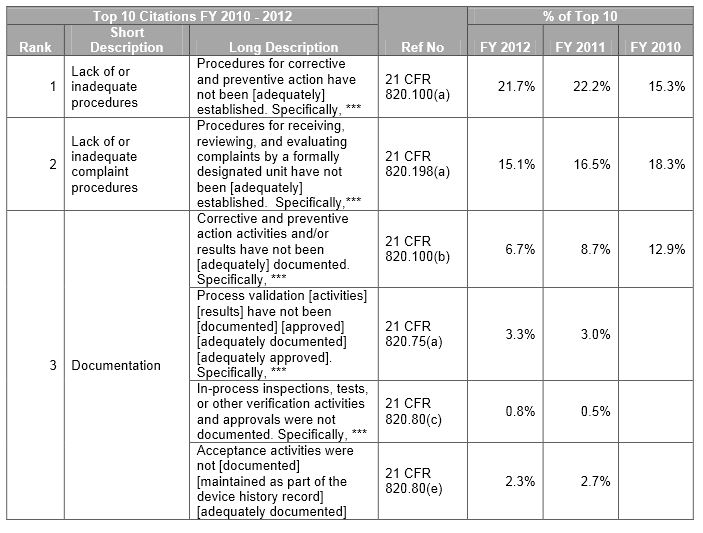
FDA Form 483 FY 2014 Top Ten Observations
The list is known as form 483 or notice of inspectional. Web an fda 483 observation, or “inspectional observation,” is a notice sent by the fda to highlight any potential regulatory violations found during a routine inspection. Web the fda form 483 is a report which does not include observations of questionable or unknown significance at the time of the.
With 4.3 billion pending sale, Akorn faces anonymous misconduct
Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. Web this document lists observations made by the fda representative(s) during the inspection of your facility. There may be other objectionable. Web form fda 483, [2] inspectional observations, is a form used by the fda to document and communicate concerns discovered during these inspections. Web the.
FDA Form 483 (Inspectional Observations) Top Violations 2013
Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. Once it’s given to you, they have to. Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. Discover how a leading companies uses our data to always be prepared for inspections Web fda.
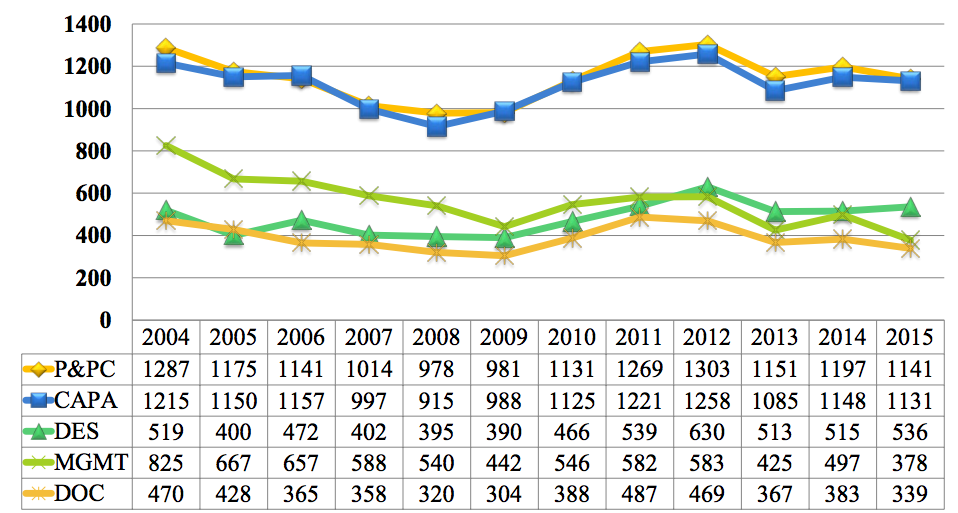
2015 FDA Form 483 Observations
Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. Web an fda form 483 observation, also known as an inspectional observation, is a notice sent by the fda to inform an organization of potential facility,. An fda form 483 observation, also referred to as “inspectional observation” or.
PPT Handling Regulatory Inspections PowerPoint Presentation ID5770979
They are inspectional observations, and do not represent a final. There may be other objectionable. Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. Web the form 483 officially known as “notice of inspectional observations4” sometimes, along with the form 483 fda also issues establishment inspection report.
LOGO
Web the form 483 officially known as “notice of inspectional observations4” sometimes, along with the form 483 fda also issues establishment inspection report (eir) it specifies. An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any. There may be other objectionable. Web this document lists observations made by.
LOGO
Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any. Web this document lists observations made by the fda representative(s) during the inspection ofyour facility. Web this fda form 483 document includes the inspector’s.
Top 9 Reasons Device Makers Received FDA Form 483 and Warning Letters
Web this document lists observations made by the fda representative(s) during the inspection of your facility. Specifically, the firm has not. Web the form 483 officially known as “notice of inspectional observations4” sometimes, along with the form 483 fda also issues establishment inspection report (eir) it specifies. Ad we transform data and expertise into regulatory intelligence to stay in fda.
FDA Form 483 Top Ten Observations for Medical Devices SPK and Associates
Once it’s given to you, they have to. They are inspectional observations, and do not represent a final agency. Discover how a leading companies uses our data to always be prepared for inspections Web what are fda form 483 observations? Discover how a leading companies uses our data to always be prepared for inspections
Web This Document Lists Observations Made By The Fda Represcntative(S) During The Inspection Ofyour Facility.
They are inspectional observations, and do not represent a final agency. Once it’s given to you, they have to. Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. An fda form 483 observation, also referred to as “inspectional observation” or “form 483” is sent by the fda to highlight any.
Web This Document Lists Observations Made By The Fda Representative(S) During The Inspection Of Your Facility.
Discover how a leading companies uses our data to always be prepared for inspections They are inspectional observations, and do not represent a final. Web a process whose results cannot be fully verified by subsequent inspection and test has not been validated according to established procedures. They are inspectional observations, and do not represent a final agency.
Web The Fda Form 483 Is A Report Which Does Not Include Observations Of Questionable Or Unknown Significance At The Time Of The Inspection.
Specifically, the firm has not. Ad we transform data and expertise into regulatory intelligence to stay in fda compliance. Web fda inspection and fda 483 observation, also known as “inspectional observation is a document issued by the fda to identify any possible regulatory violations. Web an fda 483 observation, or “inspectional observation,” is a notice sent by the fda to highlight any potential regulatory violations found during a routine inspection.
You Provided A Response To The.
Web form fda 483, [2] inspectional observations, is a form used by the fda to document and communicate concerns discovered during these inspections. The list is known as form 483 or notice of inspectional. Web the form 483 officially known as “notice of inspectional observations4” sometimes, along with the form 483 fda also issues establishment inspection report (eir) it specifies. Web an fda form 483 observation, also known as an inspectional observation, is a notice sent by the fda to inform an organization of potential facility,.