General Form Of A Rate Law
General Form Of A Rate Law - Web a rate law is any mathematical relationship that relates the concentration of a reactant or product in a chemical reaction to time. Well, you are given the mechanism for the overall reaction, so you cannot say that the rate law is r(t) = k[n o2]2[f 2]. The most general description of a chemical reaction network considers a number of distinct chemical species reacting via reactions. Web explain the form and function of a rate law. Web these are called integrated rate laws. Web a differential rate law expresses the rate of a reaction in terms of changes in the concentration of reactants over a specific interval of time. Web still, revenue from reels is growing, reaching an annual sales rate of $10 billion, zuckerberg said, up from $3 billion in the third quarter of 2022. Web what is the general form of a rate law? An equation relating the rate of a chemical reaction to the concentrations or partial pressures of the reactants. Here, learn about texas' different types of powers of attorney, including general, limited,.
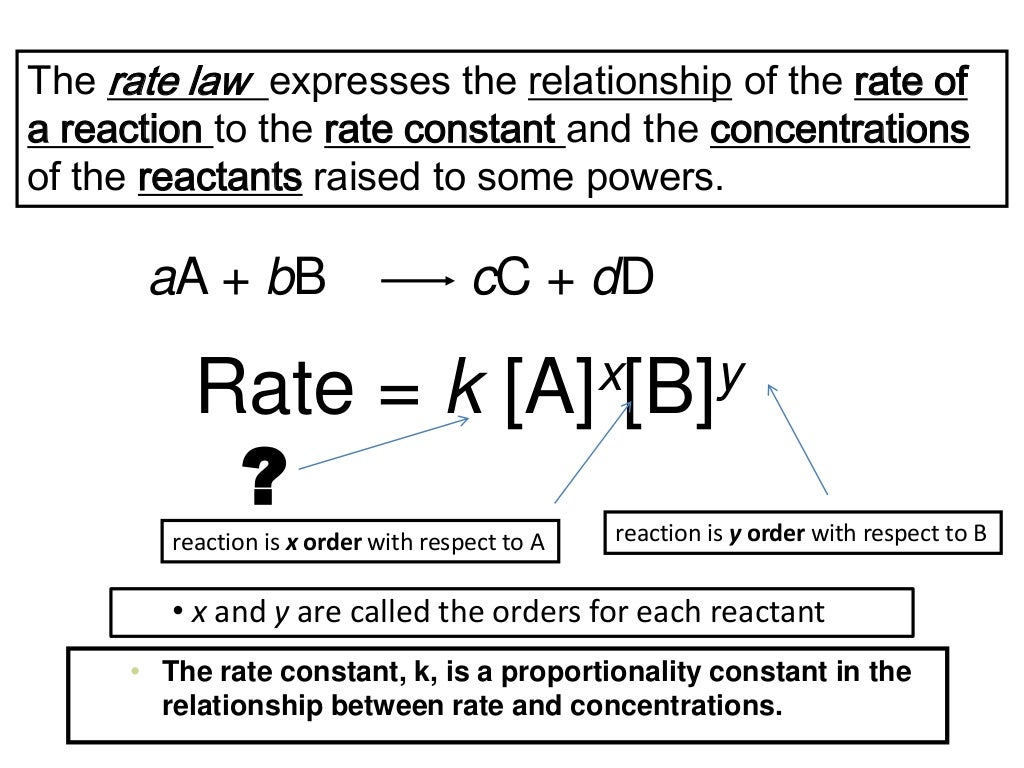
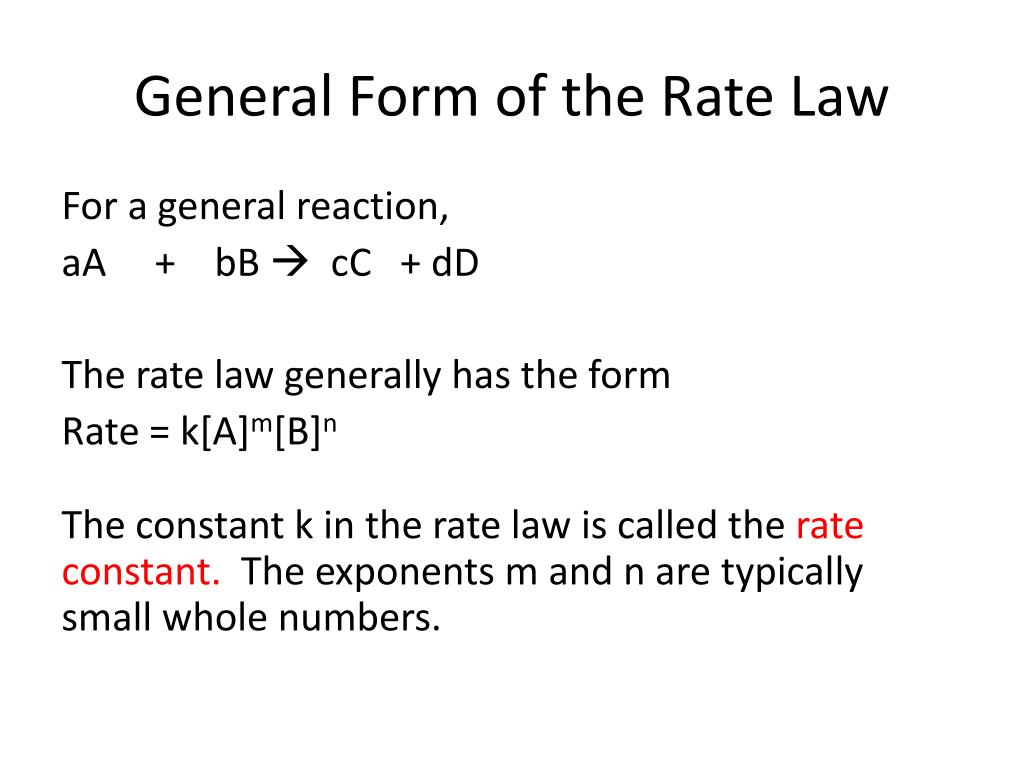
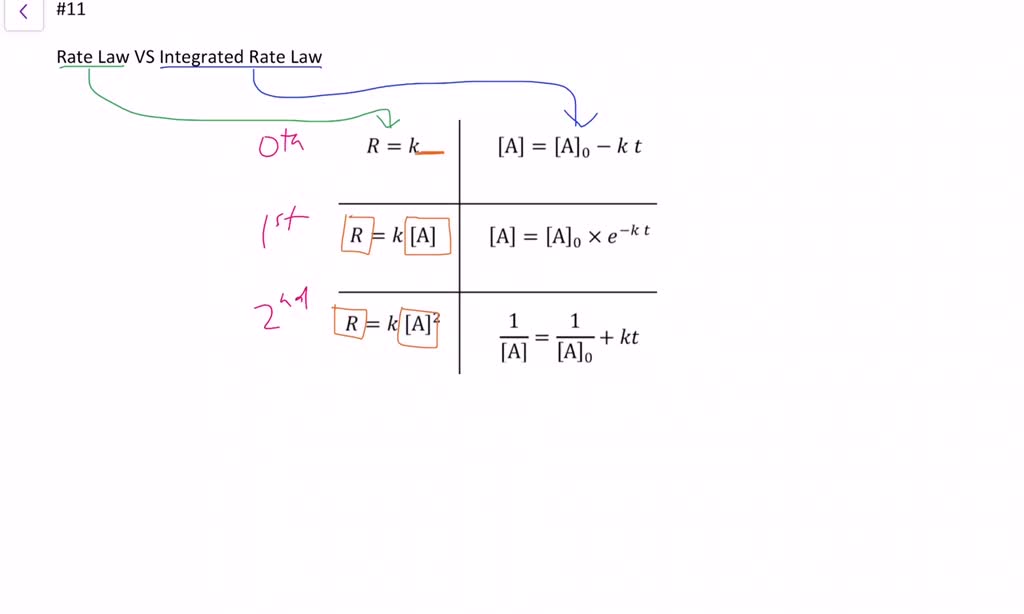
Web integrated form of the zeroth order rate law. Well, you are given the mechanism for the overall reaction, so you cannot say that the rate law is r(t) = k[n o2]2[f 2]. Web what is the general form of a rate law? We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate. Web this article provides information and answers on powers of attorney in texas. Web as of july 19, the average credit card interest rate is 20.44%, down slightly from the 20.58% recorded the week before, according to bankrate.com. Rate laws can be expressed in either. Web in the standard form, the rate law equation is written as: Is reaction rate, expressed in concentration/unit of time (usually = molarity/second) is the specific rate constant and are. Web rate laws or rate equations are mathematical expressions that describe the relationship between the rate of a chemical reaction and the concentration of its reactants.
We can use an integrated rate law to determine the amount of reactant or product present after a period of time or to estimate. Use rate laws to calculate reaction rates. The rate law for a chemical reaction is an equation that. The differential rate law is. Integration of the differential rate law yields the concentration as a function of time. Web as of july 19, the average credit card interest rate is 20.44%, down slightly from the 20.58% recorded the week before, according to bankrate.com. Web what is the general form of a rate law? Rate laws can be expressed in either. Is reaction rate, expressed in concentration/unit of time (usually = molarity/second) is the specific rate constant and are. Web still, revenue from reels is growing, reaching an annual sales rate of $10 billion, zuckerberg said, up from $3 billion in the third quarter of 2022.
Integrated Rate Law Problems 1 YouTube
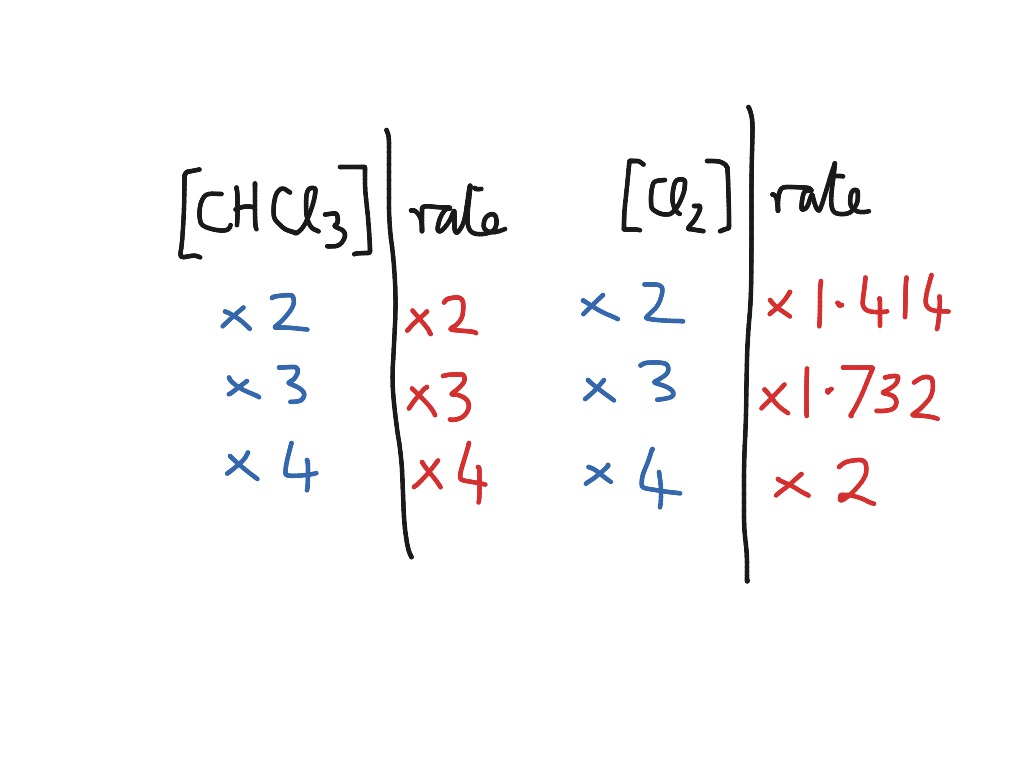
Web july 19, 2023. Which statement is true for the general rate law, rate = k[a][b] %3d a: Integration of the differential rate law yields the concentration as a function of time. Rate = k [a]^x [b]^y it is found that for the reaction a+b —> c that doubling the concentration of either a or b quadruples the rate of.
13.2 The Rate Law YouTube
Web according to the law of mass action, the rate of a chemical reaction at a constant temperature depends only on the concentrations of the substances that. Rate = k [a]^x [b]^y it is found that for the reaction a+b —> c that doubling the concentration of either a or b quadruples the rate of the. Web a rate law.
Rate Law YouTube
Web a differential rate law expresses the rate of a reaction in terms of changes in the concentration of reactants over a specific interval of time. Web the rate law of a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants. Rate law (or) rate equation: Which statement.
8.1 rate law
Rate=k [a]m [b]n [c]p… rate = k [ a ] m [ b ] n [ c ] p. Which statement is true for the general rate law, rate = k[a][b] %3d a: Web july 19, 2023. Web explain the form and function of a rate law. The differential rate law is.
8.1 rate law
Web what is the general form of a rate law? The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction. Start with the general rate law. Rate = k [a]^x [b]^y it is found that for the reaction a+b —> c that doubling the.
A rate law example Science ShowMe
Start with the general rate law. An equation relating the rate of a chemical reaction to the concentrations or partial pressures of the reactants. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction. Well, you are given the mechanism for the overall reaction,.
Solving a Rate Law Using the Initial Rates Method YouTube
Web write the genral form of the rate law for each of the following reactions this problem has been solved! The equation which gives exact mathematical relation between rate of… q:. Web a differential rate law expresses the rate of a reaction in terms of changes in the concentration of reactants over a specific interval of time. The most general.
PPT The Rate Law PowerPoint Presentation, free download ID5857354
Here, learn about texas' different types of powers of attorney, including general, limited,. Rate laws can be expressed in either. The rate law for a chemical reaction is an equation that. Use rate and concentration data to identify reaction orders and derive rate laws. Web july 19, 2023.
Introduction to the Rate Law YouTube
The differential rate law is. Web this article provides information and answers on powers of attorney in texas. Use rate laws to calculate reaction rates. Web according to the law of mass action, the rate of a chemical reaction at a constant temperature depends only on the concentrations of the substances that. We can use an integrated rate law to.
The type of rate law for a reaction, either the d…
100% (12 ratings) general form of rate law, rate = k [a]^x [b]^y where, k = ra. Web the rate law of a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants. Rate=k [a]m [b]n [c]p… rate = k [ a ] m [ b ] n [.
Web Still, Revenue From Reels Is Growing, Reaching An Annual Sales Rate Of $10 Billion, Zuckerberg Said, Up From $3 Billion In The Third Quarter Of 2022.
Web a rate law is any mathematical relationship that relates the concentration of a reactant or product in a chemical reaction to time. The rate law (also known as the rate equation) for a chemical reaction is an expression that provides a relationship between the rate of the reaction. The rate law for a chemical reaction is an equation that. Web in the standard form, the rate law equation is written as:
Web Rate Laws Or Rate Equations Are Mathematical Expressions That Describe The Relationship Between The Rate Of A Chemical Reaction And The Concentration Of Its Reactants.
100% (12 ratings) general form of rate law, rate = k [a]^x [b]^y where, k = ra. Web these are called integrated rate laws. Well, you are given the mechanism for the overall reaction, so you cannot say that the rate law is r(t) = k[n o2]2[f 2]. Rate law (or) rate equation:
Web In General, A Rate Law (Or Differential Rate Law, As It Is Sometimes Called) Takes This Form:
The differential rate law is. Integration of the differential rate law yields the concentration as a function of time. Web the rate law of a chemical reaction is an expression that provides a relationship between the rate of the reaction and the concentrations of the reactants. Which statement is true for the general rate law, rate = k[a][b] %3d a:
Is Reaction Rate, Expressed In Concentration/Unit Of Time (Usually = Molarity/Second) Is The Specific Rate Constant And Are.
Web write the genral form of the rate law for each of the following reactions this problem has been solved! Rate = k [a]^x [b]^y it is found that for the reaction a+b —> c that doubling the concentration of either a or b quadruples the rate of the. Web integrated form of the zeroth order rate law. The equation which gives exact mathematical relation between rate of… q:.