How Do Atoms Form A New Substance
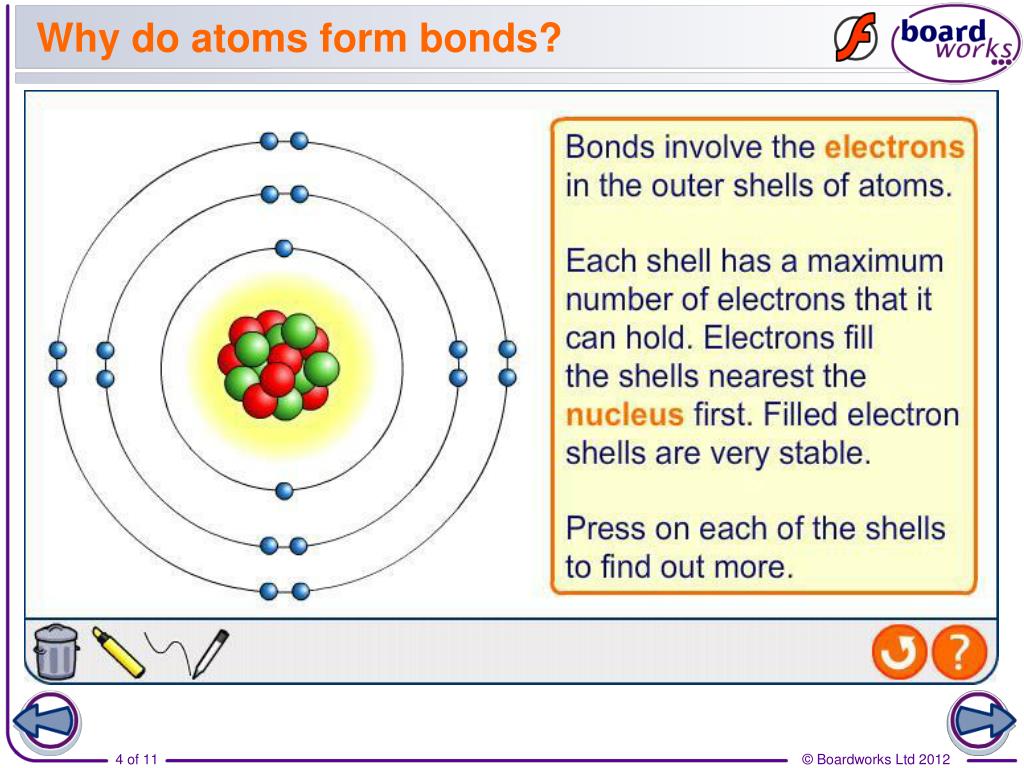
How Do Atoms Form A New Substance - It is these bonds that define what is formed. The figure below shows an example of how new substances can form. Here is einem oxidise copper lion statute in front of of chicago art institute and the aon center. Web when atoms combine to form a new substance, they are forming chemical bonds that bind them together. The atoms of molecules are linked together through a reaction known as chemical bonding. Web the process of rusting, or oxidization, exemplifies one chemical reaction. Web how do atoms form a new substance. A.by sharing electrons with each other b. Web atoms combine as the electrons from each atom are attracted to the nuclei of the atoms. The nature of the bond.
The figure below shows an example of how new substances can form. Web how do atoms form a new substance chemistry middle school how do atoms form a new substance answers answer 1 atoms form a new substance by bonding with. Atoms form a new substance by sharing electrons with each other. It is these bonds that define what is formed. Web at the surface of the earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides. Web atoms combine as the electrons from each atom are attracted to the nuclei of the atoms. For example, as we just saw, the chemical. Web remember that a physical change is a change in properties such as texture, shape, or state, while a chemical change represents the formation of a new substance. If a neutral atom loses an electron, it becomes a positive ion. If it gains an electron, it becomes a.
Atoms can also combine to. By losing electrons c.by gaining electrons from each other d. Web [show answer.] as your study of chemistry continues, you will find that sometimes chemists write molecular formulas in different ways. Gas bubbles rise in a liquid. Hydrogen and chlorine are diatomic molecules. Here is einem oxidise copper lion statute in front of of chicago art institute and the aon center. Web ordinary atoms that either gain or lose electrons are called ions. A.by sharing electrons with each other b. Web quiz course 44k views reactants and products chemical reactions make and break the chemical bonds that hold atoms together to create molecules. Web remember that a physical change is a change in properties such as texture, shape, or state, while a chemical change represents the formation of a new substance.
Why do atoms form molecules? Quantum mechanical model of atoms. Arvin
Web at the surface of the earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides. If it gains an electron, it becomes a. Web jul 26, 2022 7:48 pm edt. The nature of the bond. Web [show answer.] as your study of chemistry continues, you will find that sometimes chemists write molecular.
thinkbiggerdesigns Why Do Atoms Ions
Covalent bonds are when at least one pair of electrons are shared. This results in bonds ranging from 100% covalent to bonds with. When atoms combine to form a new substance, they are forming chemical bonds that bind them together. Web the combination of atoms to form compounds occurs when the compounds being formed are at lower energy than the.
How do atoms ‘know’ what other atoms to bond to? BBC Science Focus
Web quiz course 44k views reactants and products chemical reactions make and break the chemical bonds that hold atoms together to create molecules. Web [show answer.] as your study of chemistry continues, you will find that sometimes chemists write molecular formulas in different ways. Here is einem oxidise copper lion statute in front of of chicago art institute and the.
Why Do Atoms Form Molecules? Wonderopolis
If it gains an electron, it becomes a. By losing neutrons to each other i. Web the combination of atoms to form compounds occurs when the compounds being formed are at lower energy than the original atoms. Web quiz course 44k views reactants and products chemical reactions make and break the chemical bonds that hold atoms together to create molecules..
thinkbiggerdesigns Why Do Atoms Ions
For example, as we just saw, the chemical. If it gains an electron, it becomes a. Web how new substances form. When atoms combine to form a new substance, they are forming chemical bonds that bind them together. By losing neutrons to each other i.
PPT Molecules of Life PowerPoint Presentation, free download ID1825272
Web in a chemical change, the molecules in the reactants interact to form new substances. A.by sharing electrons with each other b. Web when atoms combine to form a new substance, they are forming chemical bonds that bind them together. If a neutral atom loses an electron, it becomes a positive ion. Hydrogen and chlorine are diatomic molecules.
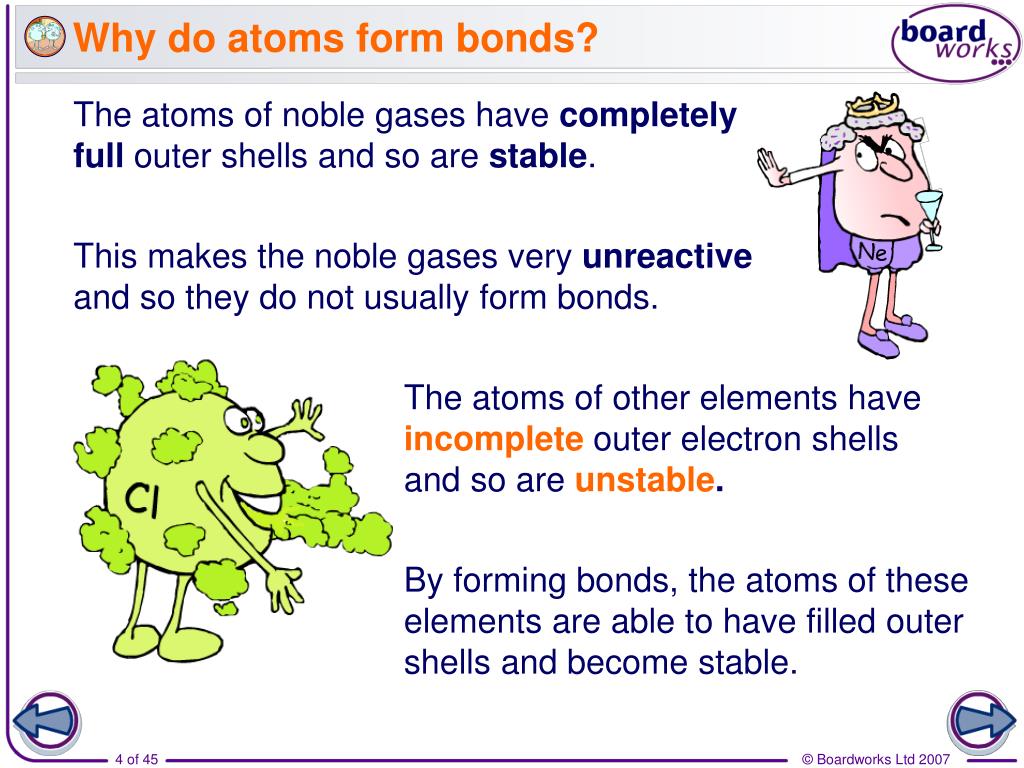
Why do atoms form bonds? YouTube
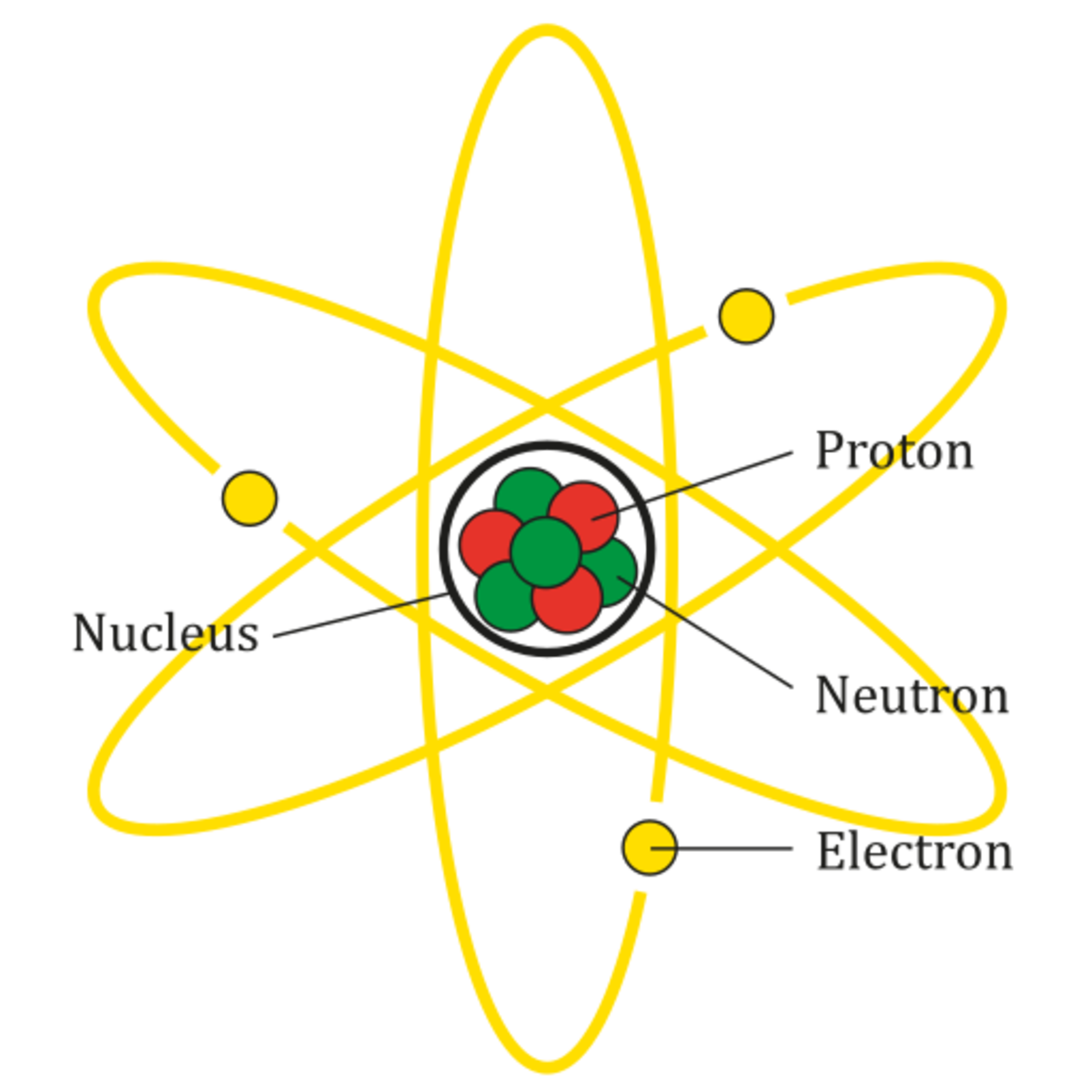
If a neutral atom loses an electron, it becomes a positive ion. Atomic structure of carbon atom showing the. By losing neutrons to each other i. Web [show answer.] as your study of chemistry continues, you will find that sometimes chemists write molecular formulas in different ways. It is these bonds that define what is formed.
why do atoms react why do atoms react with one another to form
By losing electrons c.by gaining electrons from each other d. This results in bonds ranging from 100% covalent to bonds with. Web in a chemical change, the molecules in the reactants interact to form new substances. If a neutral atom loses an electron, it becomes a positive ion. Web how new substances form.
PPT Ions PowerPoint Presentation, free download ID6738771
Web in a chemical change, the molecules in the reactants interact to form new substances. A.by sharing electrons with each other b. Web the combination of atoms to form compounds occurs when the compounds being formed are at lower energy than the original atoms. Web how do atoms form a new substance chemistry middle school how do atoms form a.
Atoms, Molecules, and Compounds What's the Difference? Owlcation
Web the process of rusting, or oxidization, exemplifies one chemical reaction. The nature of the bond. The figure below shows an example of how new substances can form. In a physical change, like a state change or dissolving, no new substance is formed. Web remember that a physical change is a change in properties such as texture, shape, or state,.
Web The Process Of Rusting, Or Oxidization, Exemplifies One Chemical Reaction.
Web at the surface of the earth, an overwhelming majority of atoms combine to form various compounds, including water, salt, silicates and oxides. By losing electrons c.by gaining electrons from each other d. A.by sharing electrons with each other b. Gas bubbles rise in a liquid.
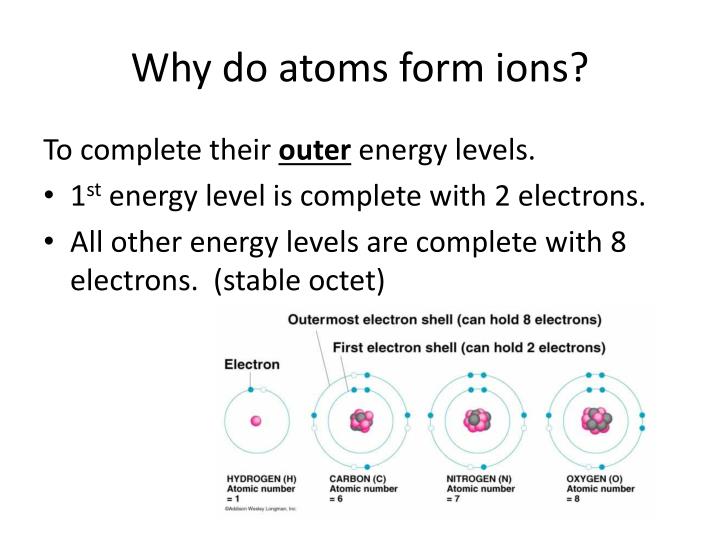
If A Neutral Atom Loses An Electron, It Becomes A Positive Ion.
Web when atoms combine to form a new substance, they are forming chemical bonds that bind them together. Hydrogen and chlorine are diatomic molecules. Web how do atoms form a new substance? The figure below shows an example of how new substances can form.
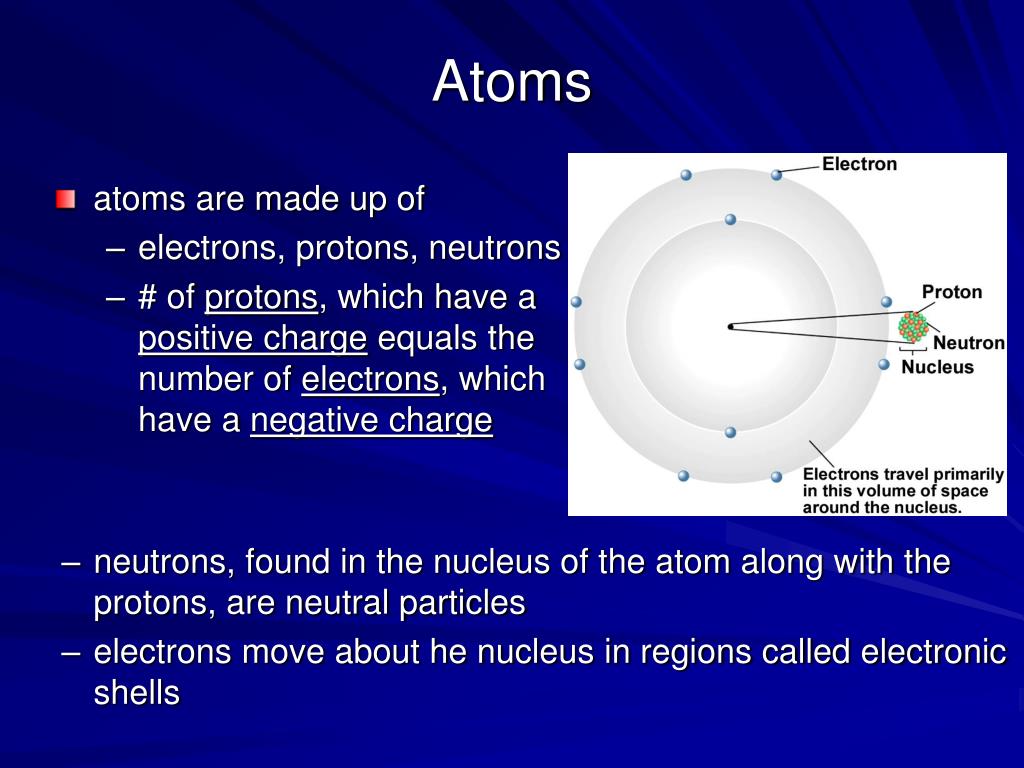
Web Atoms Combine As The Electrons From Each Atom Are Attracted To The Nuclei Of The Atoms.
When atoms combine to form a new substance, they are forming chemical bonds that bind them together. This results in bonds ranging from 100% covalent to bonds with. For example, as we just saw, the chemical. Web ordinary atoms that either gain or lose electrons are called ions.
The Nature Of The Bond.
The atoms of molecules are linked together through a reaction known as chemical bonding. In a physical change, like a state change or dissolving, no new substance is formed. Covalent bonds are when at least one pair of electrons are shared. If it gains an electron, it becomes a.