How Many Bonds Does Bromine Form
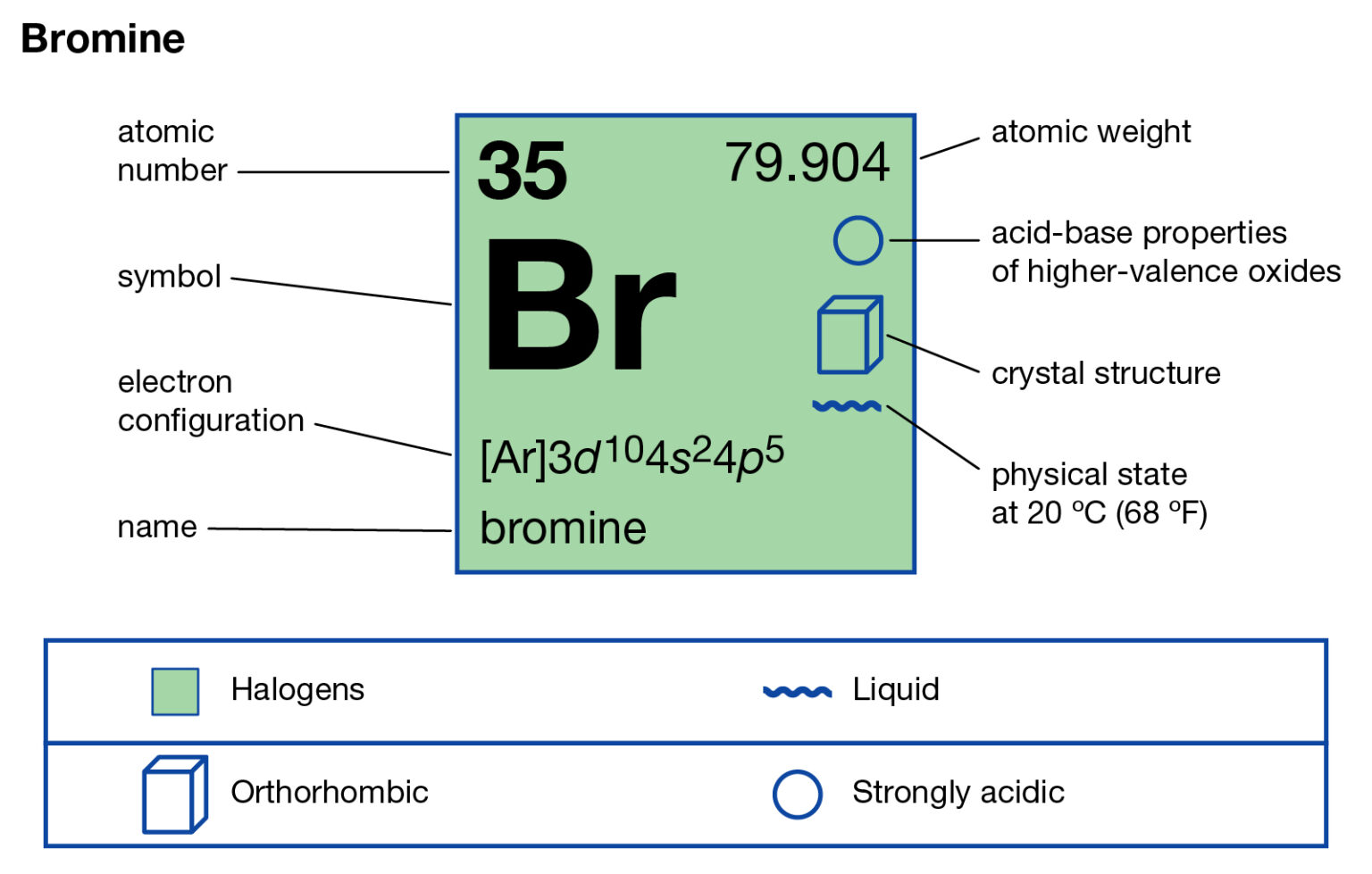
How Many Bonds Does Bromine Form - Bromine atoms have a strong. It can form 1, 2, or 3 bonds with other atoms. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Chemical element in the periodic table of elements. A colorless gas, it dissolves in. Hydrogen bromide is the inorganic compound with the formula h br. Molybdenum bromine oxygen potassium nitrogen how many single covalent bonds does each element. Its companions include fluorine, chlorine, and iodine. Which of the following situations meet the bonding requirement for carbon atoms. Bromine is a halogen and has 7 valence electrons.
It is a hydrogen halide consisting of hydrogen and bromine. 0 how many bonds will a carbon atom form with four bromine atoms? Bromine is a halogen and has 7 valence electrons. 6 which elements tend to form covalent. It has 35 protons and 35 electrons in the atomic structure. Two single bonds and a double bond. Web how many covalent bonds will bromine and iodine form? Hydrogen bromide is the inorganic compound with the formula h br. They will form seven bonds along with all the other elements in that column on the periodic table. Modeling lonic and covalent bonds part 1:
Modeling lonic and covalent bonds part 1: Like the other halogens, bromine has seven electrons in its outer. •br br submit your choice. 0 how many bonds will a carbon atom form with four bromine atoms? Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Two single bonds and a double bond. The atomic number of bromine is 35, and the atomic weight is 79.904. Web bromine will normally form one covalent bond. Web bromine is a 35. Web how many bonds does bromine form?
HONC 1234 ChemSimplified
Web bromine will normally form one covalent bond. The maximum number of bonds. The chemical symbol for bromine is. 0 how many bonds will a carbon atom form with four bromine atoms? Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence.
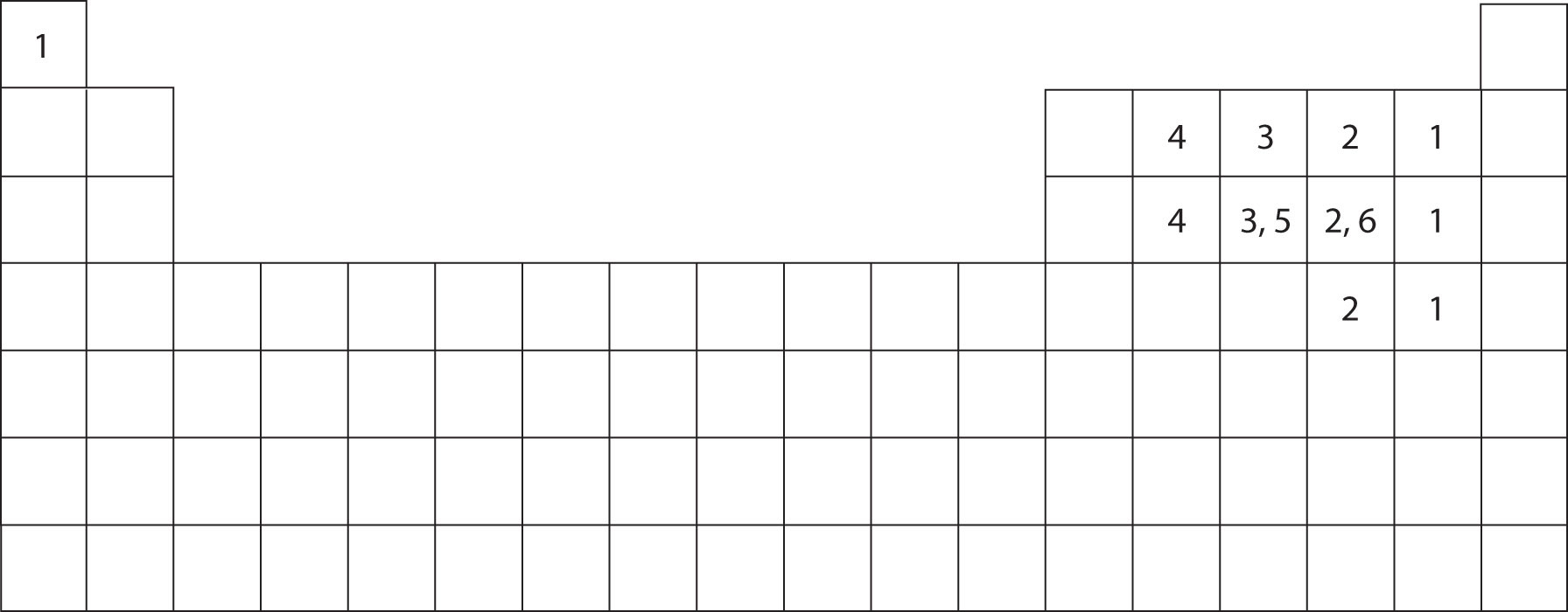
How to Predict number of bonds each element forms ChemSimplified
The atomic number of bromine is 35, and the atomic weight is 79.904. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Bromine atoms have a strong. A colorless gas, it dissolves in. Bromine is a halogen and has 7 valence electrons.
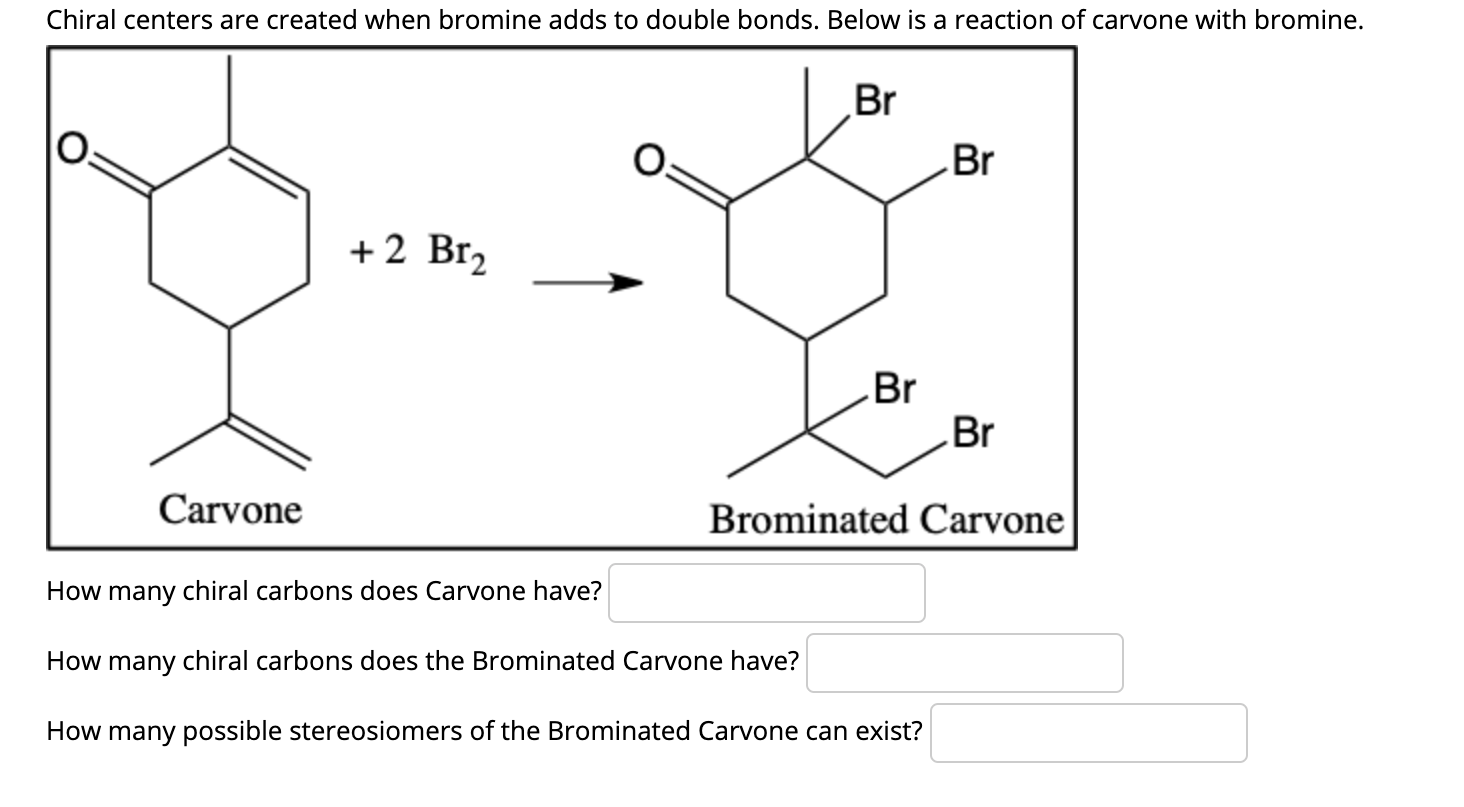
Solved Chiral centers are created when bromine adds to
A colorless gas, it dissolves in. Web how many covalent bonds will bromine and iodine form? Web it is a halogen and has an atomic symbol br. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. 0 how many bonds will a carbon atom form with four bromine atoms?
How Many Bonds Does Bromine Form? Answer]
Hydrogen bromide is the inorganic compound with the formula h br. It is the process of two or more atoms coming together. Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Bromine is a halogen and has 7 valence electrons. Modeling lonic and covalent bonds part 1:
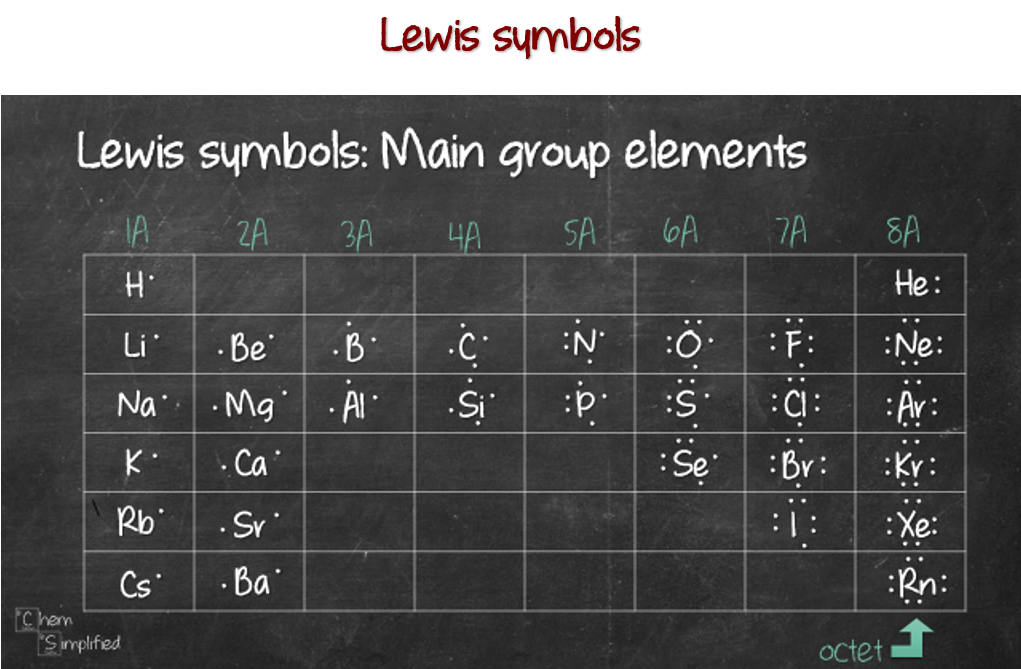
Bromine Valence Electrons Bromine Valency (Br) Dot Diagram
Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Which of the following situations meet the bonding requirement for carbon atoms. Web how many covalent bonds will bromine and iodine form? 6 which elements tend to form covalent. It has 35 protons and 35 electrons in the atomic structure.
Bromine Protons Neutrons Electrons Electron Configuration
Chemical element in the periodic table of elements. Bromine is a halogen and has 7 valence electrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols, videos and images. Its companions include fluorine, chlorine, and iodine. They will form seven bonds along with all the other elements in that column on the periodic table.
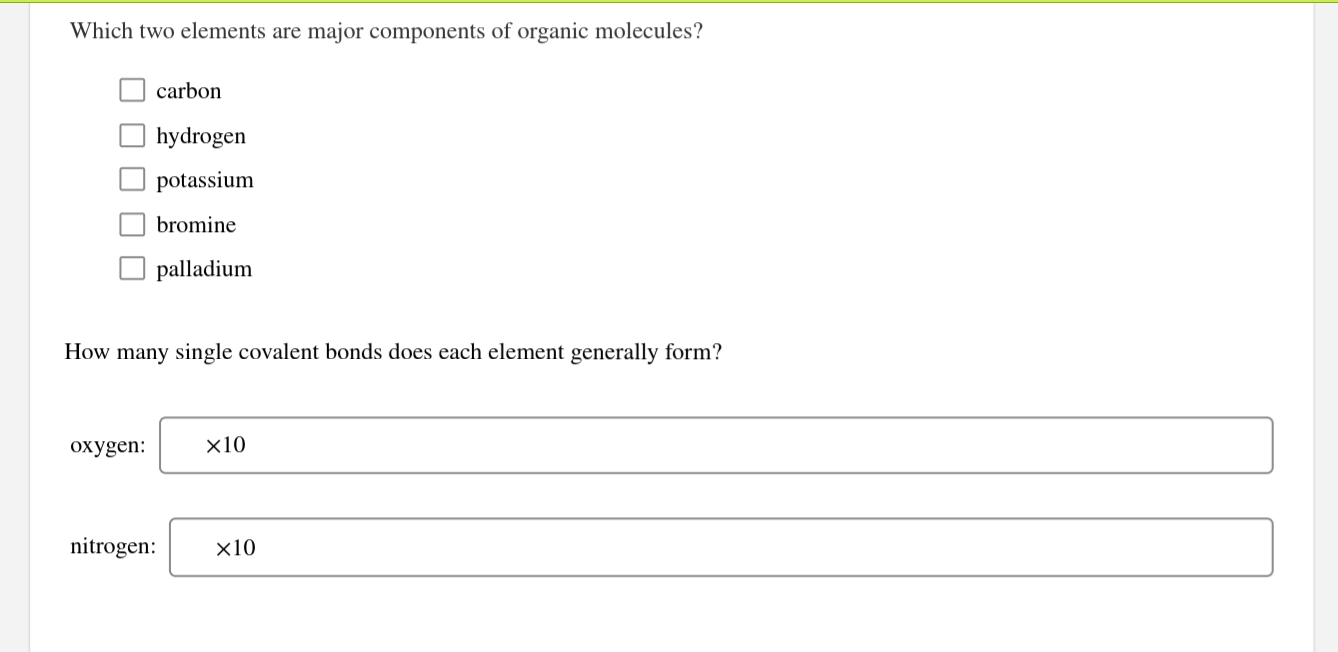
Solved Which two elements are major components of organic
Web the circles show how the valence electron shells are filled for both atoms. Web bromine will normally form one covalent bond. Bromine is a halogen and has 7 valence electrons. 6 which elements tend to form covalent. Two single bonds and a double bond.
how many bonds does sulfur form
•br br submit your choice. A colorless gas, it dissolves in. Web bromine is a 35. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. Bromine is a halogen and has 7 valence electrons.
How Many Bonds Does Nitrogen Form tour bous
Web posted sep 14, 2022. Web bromine is a 35. Hydrogen bromide is the inorganic compound with the formula h br. It has 35 protons and 35 electrons in the atomic structure. Molybdenum bromine oxygen potassium nitrogen how many single covalent bonds does each element.
Collins New GCSE Science Gateway B page 112
Two single bonds and a double bond. Bromine is a halogen and has 7 valence electrons. Web it is a halogen and has an atomic symbol br. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group. It is the process of two or more atoms coming together.
6 Which Elements Tend To Form Covalent.
Web it is a halogen and has an atomic symbol br. The chemical symbol for bromine is. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only needs one more electron to complete an octet. It can form 1, 2, or 3 bonds with other atoms.
Web The Circles Show How The Valence Electron Shells Are Filled For Both Atoms.
Web bromine is a member of the halogen family of elements. A single bond and two double bonds. Like the other halogens, bromine has seven electrons in its outer. Web bromine exists as a diatomic molecule with the chemical formula br 2 that belongs to the halogen group.
Hydrogen Bromide Is The Inorganic Compound With The Formula H Br.
Web bromine will normally form one covalent bond. Molybdenum bromine oxygen potassium nitrogen how many single covalent bonds does each element. Web how many bonds does bromine form? •br br submit your choice.
Web How Many Covalent Bonds Will Bromine And Iodine Form?
Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all seven valence electrons to reveal the next Bromine, which belongs to group 17 and period four of the periodic table, has seven outer shell or valence Bromine atoms have a strong. Which of the following situations meet the bonding requirement for carbon atoms.

![How Many Bonds Does Bromine Form? Answer]](https://images.pexels.com/photos/6373491/pexels-photo-6373491.jpeg?auto=compress&cs=tinysrgb&w=1200)