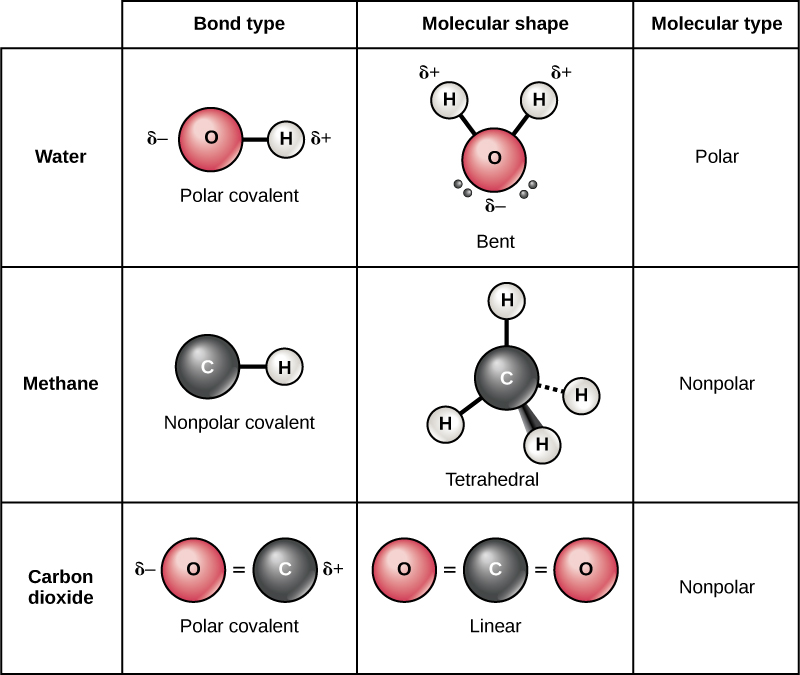
How Many Covalent Bonds Does Oxygen Form
How Many Covalent Bonds Does Oxygen Form - Web in order to form a covalent bond, each element has to share one unpaired electron. It is easier for an oxygen atom to accept or share two electrons instead of. Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic. Web atoms of different elements. Will form either one, two, three or four covalent bonds with other atoms. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. Web oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Don’t get confused as in this question single oxygen atom (o) is asked not for dioxygen o2 o 2. Each oxygen atom can form two covalent bonds. Web oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds:
Web chemistry chemistry questions and answers how many covalent bonds does an oxygen atom typically form? Web oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Web the first oxygen has three bonds, the second only has one. Calculating the number of covalent bonds possible from the number of valence electrons chemistry the valence shell of oxygen is the second. The single electrons match up to make pairs (fig. Web what is the maximum number of covalent bonds that an oxygen atom can form? Web oxygen normally will form two covalent bonds, as for example in the most familiar compound, water, in which one oxygen atom forms a covalent bond with each. You can think of the reaction taking place by a lone pair on the oxygen of one water molecule ripping off. Web in order to form a covalent bond, each element has to share one unpaired electron. The oxygen atom forms two.
Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic. Web oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: Web in order to form a covalent bond, each element has to share one unpaired electron. The number of electrons required to obtain an octet determines the number of. Web oxygen normally will form two covalent bonds, as for example in the most familiar compound, water, in which one oxygen atom forms a covalent bond with each. Don’t get confused as in this question single oxygen atom (o) is asked not for dioxygen o2 o 2. Will form either one, two, three or four covalent bonds with other atoms. Web what is the maximum number of covalent bonds that an oxygen atom can form? Web oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. There is a quick way to work out how many covalent bonds an element will.
Covalent Bonding (Biology) — Definition & Role Expii
It is easier for an oxygen atom to accept or share two electrons instead of. Two oxygen atoms must share 2. You can think of the reaction taking place by a lone pair on the oxygen of one water molecule ripping off. Fluorine and the other halogens in group 7a (17) have seven valence. Thus, bonding in potassium nitrate is.
Is SiO2 Ionic or Covalent? Techiescientist
The number of electrons required to obtain an octet determines the number of. Web oxygen normally will form two covalent bonds, as for example in the most familiar compound, water, in which one oxygen atom forms a covalent bond with each. Web chemistry chemistry questions and answers how many covalent bonds does an oxygen atom typically form? You can think.
Atoms, Isotopes, Ions, and Molecules The Building Blocks · Biology
Each oxygen atom can form two covalent bonds. Web therefore, oxygen can form 2 single covalent bonds. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. Web atoms of different elements. Web what is the maximum number of covalent bonds that an oxygen atom can form?
Covalent Bond Biology Dictionary
Thus, bonding in potassium nitrate is ionic, resulting from the electrostatic. There is a quick way to work out how many covalent bonds an element will. Web oxygen can also form covalent bonds, however, it needs a further 2 electrons to complete its valence shell (it has 6). Each oxygen atom can form two covalent bonds. You can think of.
Covalent bonding in an oxygen molecule. Covalent bonding, Chemistry
The number of electrons required to obtain an octet determines the number of. Web the first oxygen has three bonds, the second only has one. Calculating the number of covalent bonds possible from the number of valence electrons chemistry the valence shell of oxygen is the second. Web oxygen and other atoms in group 6a (16) obtain an octet by.
Online Essay Help amazonia.fiocruz.br
The number of electrons required to obtain an octet determines the number of. Web oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: There is a quick way to work out how many covalent bonds an element will. Calculating the number of covalent bonds possible from the number of valence electrons chemistry the valence.
LabXchange
The number of electrons required to obtain an octet determines the number of. It is easier for an oxygen atom to accept or share two electrons instead of. Use your own words, don't copy and paste. Web chemistry chemistry questions and answers how many covalent bonds does an oxygen atom typically form? Web atoms of different elements.
PPT KS4 Chemistry PowerPoint Presentation, free download ID2755080
Web oxygen can also form covalent bonds, however, it needs a further 2 electrons to complete its valence shell (it has 6). Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion. The number of electrons required to obtain an octet determines the number of. You can think of the reaction.
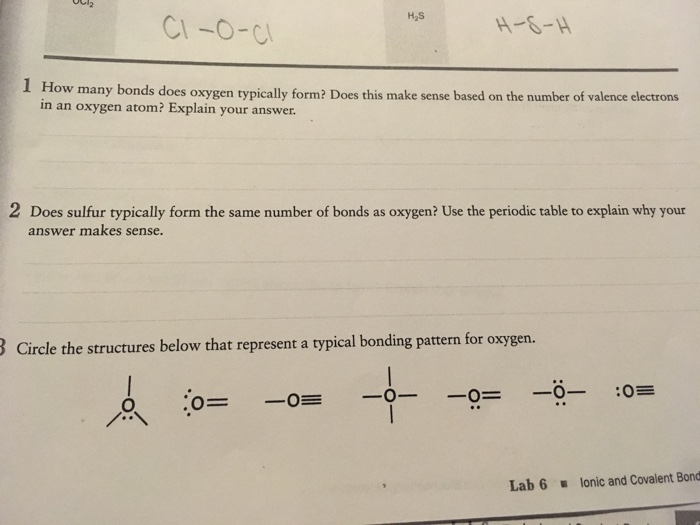
Solved H2S CIOC HSH 1 How many bonds does oxygen
Don’t get confused as in this question single oxygen atom (o) is asked not for dioxygen o2 o 2. Will form either one, two, three or four covalent bonds with other atoms. There is a quick way to work out how many covalent bonds an element will. It is easier for an oxygen atom to accept or share two electrons.
How Many Single Bonds Can Carbon Form fredhughesdesign
Fluorine and the other halogens in group 7a (17) have seven valence. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. It is easier for an oxygen atom to accept or share.
Web Oxygen And Other Atoms In Group 6A (16) Obtain An Octet By Forming Two Covalent Bonds.
Web oxygen and other atoms in group 16 obtain an octet by forming two covalent bonds: Web chemistry chemistry questions and answers how many covalent bonds does an oxygen atom typically form? Web oxygen can also form covalent bonds, however, it needs a further 2 electrons to complete its valence shell (it has 6). The single electrons match up to make pairs (fig.
Web Atoms Of Different Elements.
Each oxygen atom can form two covalent bonds. Web what is the maximum number of covalent bonds that an oxygen atom can form? You can think of the reaction taking place by a lone pair on the oxygen of one water molecule ripping off. Web for example, potassium nitrate, kno 3, contains the k + cation and the polyatomic no 3 − anion.
There Is A Quick Way To Work Out How Many Covalent Bonds An Element Will.
Web in order to form a covalent bond, each element has to share one unpaired electron. Web therefore, oxygen can form 2 single covalent bonds. Web oxygen can form two single bonds because it has six valent electrons on its outer shell. Web the first oxygen has three bonds, the second only has one.
Use Your Own Words, Don't Copy And Paste.
The number of electrons required to obtain an octet determines the number of. Fluorine and the other halogens in group 7a (17) have seven valence. Web oxygen forms two single covalent bonds, carbon forms four single covalent bonds and hydrogen forms one single covalent bond. Calculating the number of covalent bonds possible from the number of valence electrons chemistry the valence shell of oxygen is the second.