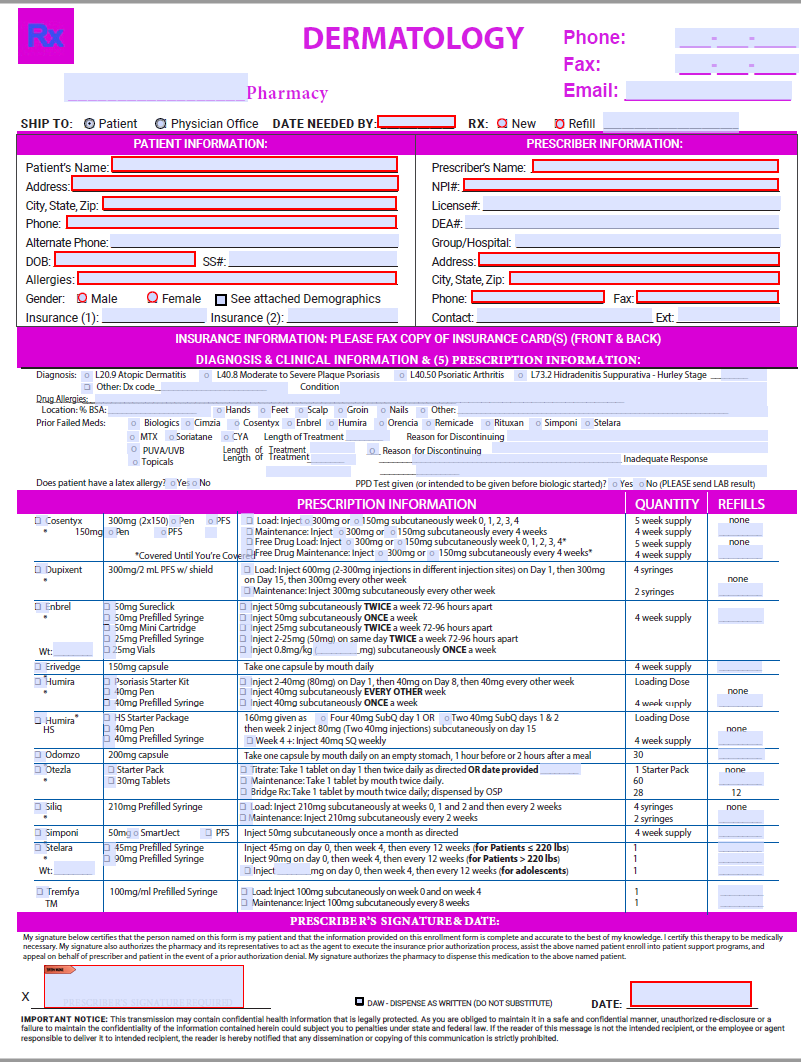
Olumiant Enrollment Form Dermatology
Olumiant Enrollment Form Dermatology - Visit the official patient site to learn more about olumiant. Find resources and support for your patients prescribed litfulo®. Visit the official patient site to learn more about olumiant. All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3. Web on june 13, the us food and drug administration (fda) approved oral baricitinib (olumiant) tablets for the treatment of severe alopecia areata in adults, which. Food and drug administration approved olumiant (baricitinib) oral tablets to treat adult patients with. Ad purpose & safety summary with warnings. Web how to make a dermatology appointment. Jak inhibitors, jak/stat pathway, atopic dermatitis, psoriasis, vitiligo, alopecia areata. Web we would like to show you a description here but the site won’t allow us.
Web patient enrollment section olumiant® (baricitinib)rheumatology updated 12/2022 office: Web initial authorization olumiant will be approved based on all of the following criteria: Food and drug administration approved olumiant (baricitinib) oral tablets to treat adult patients with. Providers can complete and submit the report online; Ad purpose & safety summary with warnings. Services provided by university health truman medical center. Ad view prescribing info, safety info & boxed warning. Web up to $40 cash back dermatology specialists of kansas city, pc for any services furnished to me by their physicians or nurse practitioner. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3.
Ad purpose & safety summary with warnings. Web on june 13, the us food and drug administration (fda) approved oral baricitinib (olumiant) tablets for the treatment of severe alopecia areata in adults, which. Ad purpose & safety summary with warnings. Web olumiant is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor. Food and drug administration (fda) for adults with severe alopecia areata (aa) (1). Web the approval of oluminant reportedly marks the first time the fda approved a “systemic treatment” for this form of alopecia. Visit the official patient site to learn more about olumiant. Ad view litfulo™ prescribing info, safety info & boxed warning on the official hcp site. Web up to $40 cash back dermatology specialists of kansas city, pc for any services furnished to me by their physicians or nurse practitioner. Providers can complete and submit the report online;
Dermatology Enrollment Form Rx Life by Anita
Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Web the recommended dose of olumiant in patients taking strong organic anion transporter 3 (oat3) inhibitors, such as probenecid, is 1 mg once daily [see drug interactions (7.1). All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office.
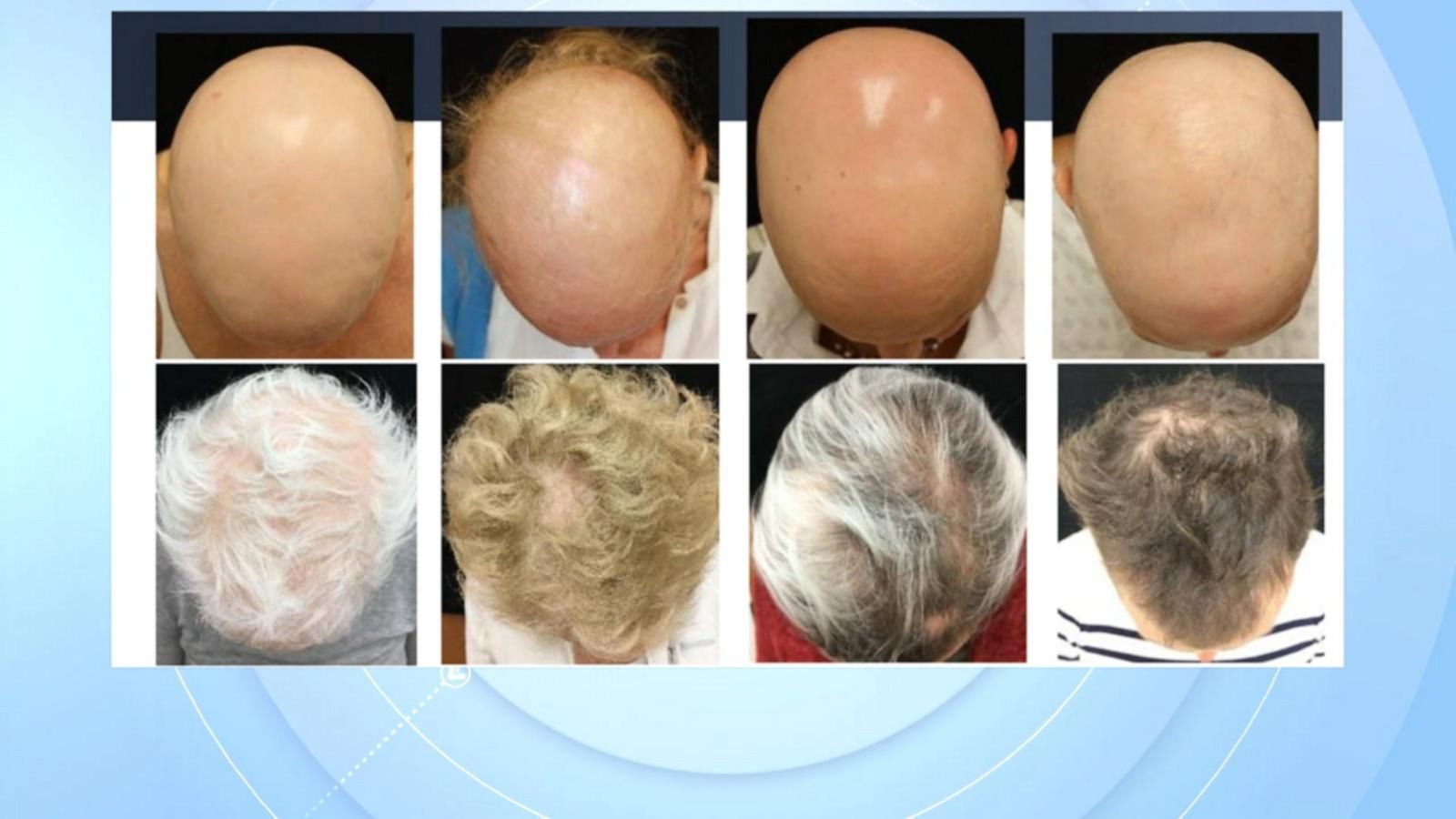
FDA approves use of Olumiant to help treat severe alopecia areata
Providers can complete and submit the report online; Web the recommended dose of olumiant in patients taking strong organic anion transporter 3 (oat3) inhibitors, such as probenecid, is 1 mg once daily [see drug interactions (7.1). Olumiant should not be given to patients with active tuberculosis.patients, except. Visit the official patient site to learn more about olumiant. Web up to.
FDA approves Olumiant to treat severe cases of alopecia areata
Visit the official patient site to learn more about olumiant. Web how to make a dermatology appointment. Web • active tuberculosis, which may present withpulmonary or extrapulmonary disease. Download support resources, including a doctor discussion guide. Web olumiant is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to.
OLUMIANT (Baricitinib) dosage, indication, interactions, side effects
All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3. Web olumiant (27.8%) compared to placebo (30.5%), but this effect was not statistically significant. Web up to $40 cash back dermatology specialists of kansas city, pc for any services furnished to me by their physicians or nurse practitioner. Olumiant should not.
About Hair Having it, losing it, and Olumiant... SINY Dermatology
Web olumiant is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Jak inhibitors, jak/stat pathway, atopic dermatitis, psoriasis, vitiligo, alopecia areata. Food and drug administration approved olumiant (baricitinib) oral.
Dermatology Referral Form Dermatology Referral Information
Download support resources, including a doctor discussion guide. Ad view prescribing info, safety info & boxed warning. Web olumiant (27.8%) compared to placebo (30.5%), but this effect was not statistically significant. Services provided by university health truman medical center. Web up to $40 cash back dermatology specialists of kansas city, pc for any services furnished to me by their physicians.
59206Optum Oncology Enrollment Form Fill Out and Sign Printable PDF
Providers can complete and submit the report online; Ad purpose & safety summary with warnings. Visit the official patient site to learn more about olumiant. Find resources and support for your patients prescribed litfulo®. In addition to our dermatologist clinic at university health, our dermatology specialists also see patients at our clinic in.
Another ‘miracle drug’ for COVID19 increasingly hard to find in
Ad purpose & safety summary with warnings. In addition to our dermatologist clinic at university health, our dermatology specialists also see patients at our clinic in. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. Download support resources, including a doctor discussion guide. Providers can complete and submit the report online;
FDA Approves Eli Lilly's Drug Olumiant For Alopecia Dermatology
Web patient enrollment section olumiant® (baricitinib)rheumatology updated 12/2022 office: All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3. Web olumiant (27.8%) compared to placebo (30.5%), but this effect was not statistically significant. Ad view prescribing info, safety info & boxed warning. Download support resources, including a doctor discussion guide.
Baricitinib Tablets Olumiant Tablet, Barinat Tablet Manufacturers
Ad purpose & safety summary with warnings. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s. I authorize any holder of medical information. Web on june 13, the us food and drug administration (fda) approved oral baricitinib (olumiant) tablets for the treatment of severe alopecia areata in adults, which. Web how to make a dermatology.
Food And Drug Administration (Fda) For Adults With Severe Alopecia Areata (Aa) (1).
Web the approval of oluminant reportedly marks the first time the fda approved a “systemic treatment” for this form of alopecia. Olumiant should not be given to patients with active tuberculosis.patients, except. Providers can complete and submit the report online; Web • active tuberculosis, which may present withpulmonary or extrapulmonary disease.
Ad Purpose & Safety Summary With Warnings.
Web how to make a dermatology appointment. Web patient enrollment section olumiant® (baricitinib)rheumatology updated 12/2022 office: Visit the official patient site to learn more about olumiant. Web olumiant (baricitinib) is an oral janus kinase (jak) inhibitor approved by the u.s.
Food And Drug Administration Approved Olumiant (Baricitinib) Oral Tablets To Treat Adult Patients With.
Jak inhibitors, jak/stat pathway, atopic dermatitis, psoriasis, vitiligo, alopecia areata. Web the recommended dose of olumiant in patients taking strong organic anion transporter 3 (oat3) inhibitors, such as probenecid, is 1 mg once daily [see drug interactions (7.1). Web initial authorization olumiant will be approved based on all of the following criteria: All olumiant coverage authorization request forms should be completed and submitted to the plan by the hcp’s office 3.
Ad View Prescribing Info, Safety Info & Boxed Warning.
Visit the official patient site to learn more about olumiant. Official patient site for litfulo™. In addition to our dermatologist clinic at university health, our dermatology specialists also see patients at our clinic in. Web olumiant is indicated for the treatment of adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor.