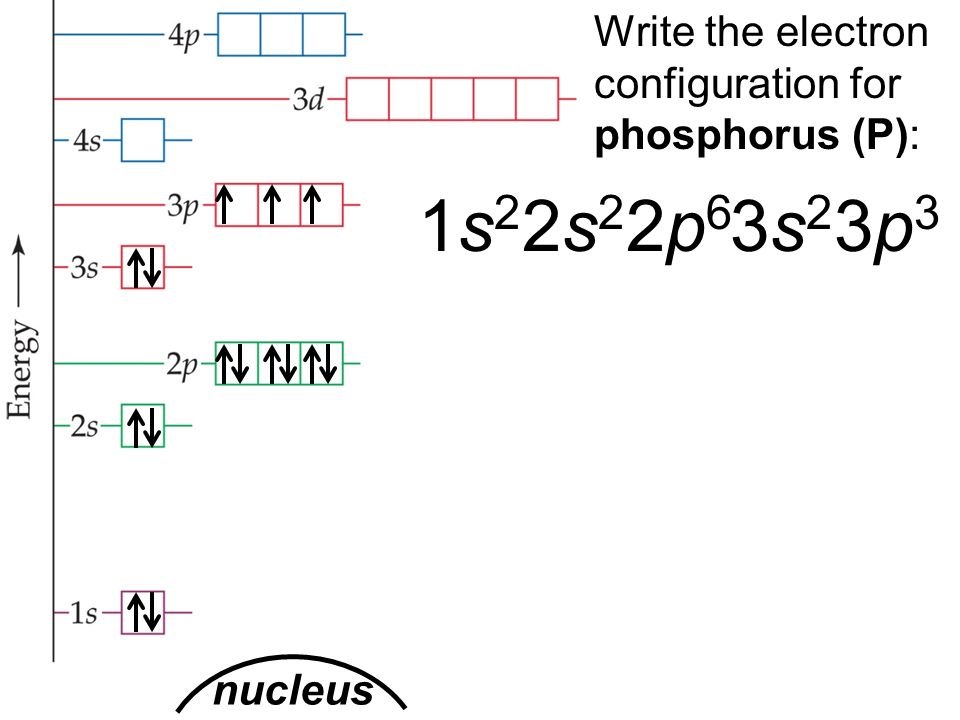
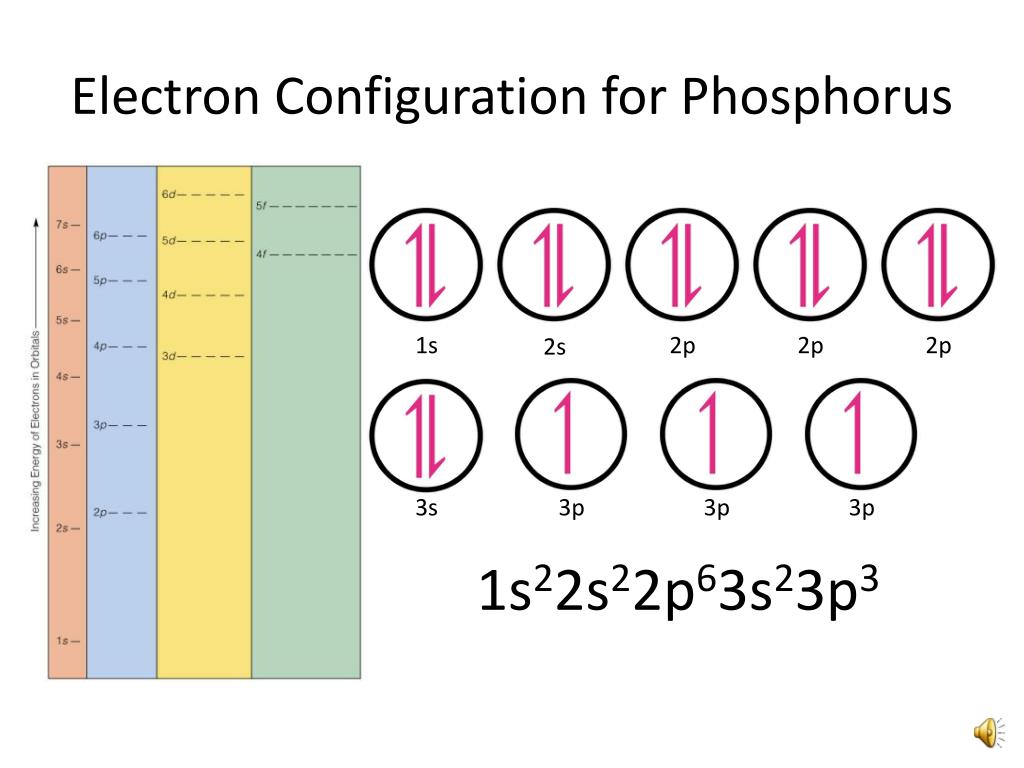
Phosphorus Electron Configuration Long Form
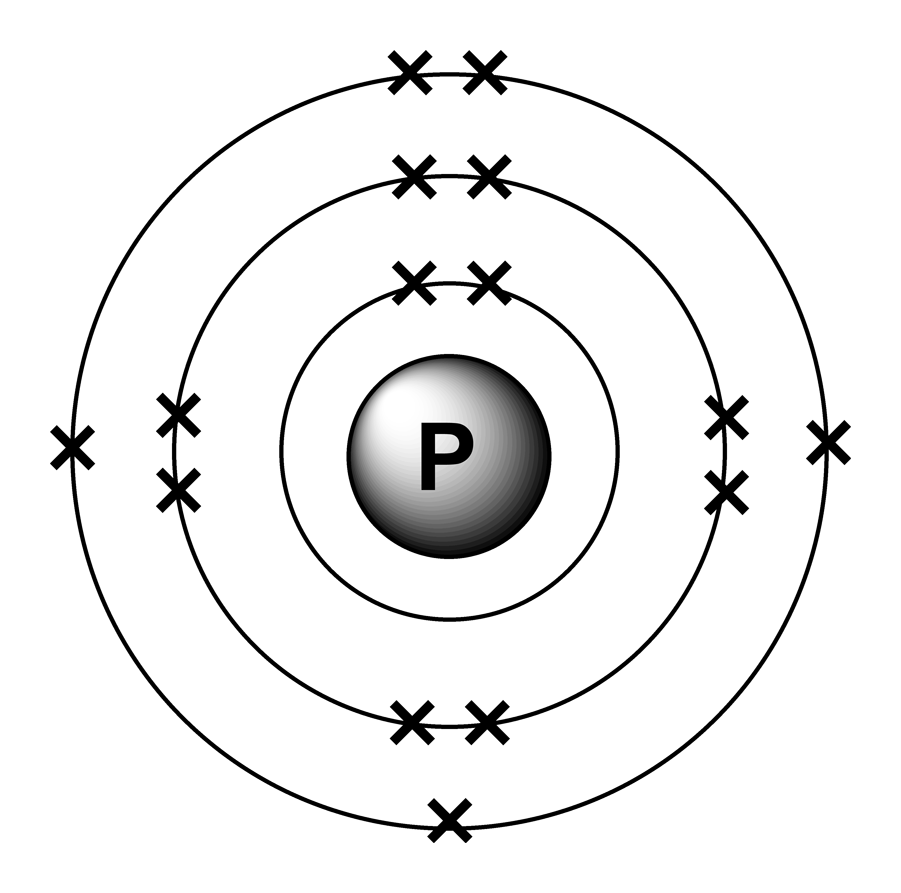
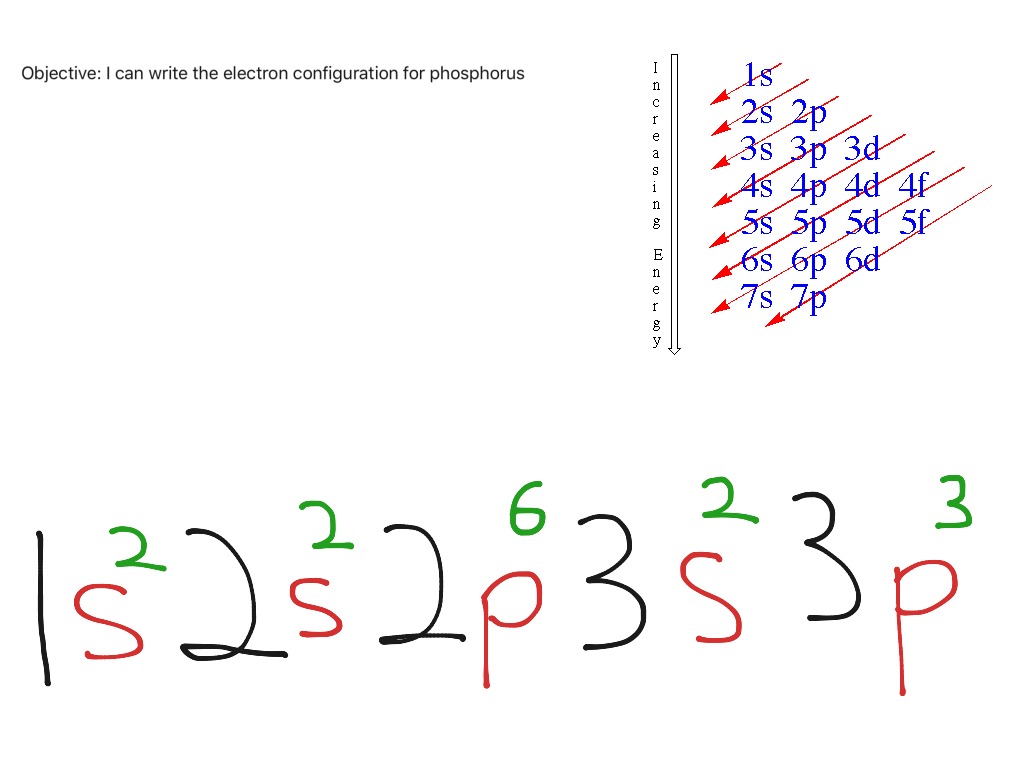
Phosphorus Electron Configuration Long Form - Web phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. The role of phosphorus is extremely significant in. The distribution of electrons are as 2 electrons in 1s subshell, electrons in 2s subshell,6 electrons in 2p subshell, 2 electrons in 3s subshell and the remaining 3 electrons in 3p subshell. Web based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. P (phosphorus) is an element with position number 15 in the periodic table. It is in period 3 and in group 5 of the periodic table. Web thus, the electron configuration of neutral phosphorus atoms is 1s 2 2s 2 2p 6 3s 2 3p 3. Web electron configuration 3s 2 3p 3: For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Electronic configuration of the phosphorus atom:
Figure 6.24 depicts how these two. It is known that elemental phosphorous occurs mostly in two forms, white and red phosphorous. Write the long form electron configuration for phosphorous. 1s 2 2s 2 2p 1: Electron configuration of boron (b) [he] 2s 2 2p 1: Electron configuration of nitrogen (n) [he] 2s 2 2p 3: It is in period 3 and in group 5 of the periodic table. 317.3 k (44.15 °c, 111.5 °f) red: The role of phosphorus is extremely significant in. Electronic configuration of chlorine atoms using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral chlorine atom.
Its boiling point is 550 degrees kelvin and its melting point is 317.3 degrees. Group 15 elements are also known as pnictogens. Electron configuration of boron (b) [he] 2s 2 2p 1: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. P (phosphorus) is an element with position number 15 in the periodic table. Web based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. 1s 2 2s 2 2p 3: 1s 2 2s 2 2p 1: ∼860 k (∼590 °c, ∼1090 °f) boiling point: That gives a total of 5 valence electrons.
Load Wiring Carbon Molecular Orbital Diagram
Some are hard to memorise (or predict), so what is the electron configuration of an atom of p? Expert solution step by step solved in 2 steps with 2 images It is known that elemental phosphorous occurs mostly in two forms, white and red phosphorous. Its boiling point is 550 degrees kelvin and its melting point is 317.3 degrees. In.
Phosphorus P (Element 15) of Periodic Table Elements FlashCards
∼860 k (∼590 °c, ∼1090 °f) boiling point: The distribution of electrons are as 2 electrons in 1s subshell, electrons in 2s subshell,6 electrons in 2p subshell, 2 electrons in 3s subshell and the remaining 3 electrons in 3p subshell. It is known that elemental phosphorous occurs mostly in two forms, white and red phosphorous. Web thus, the electron configuration.
a) The electron configuration of the outer layer for the phosphorus
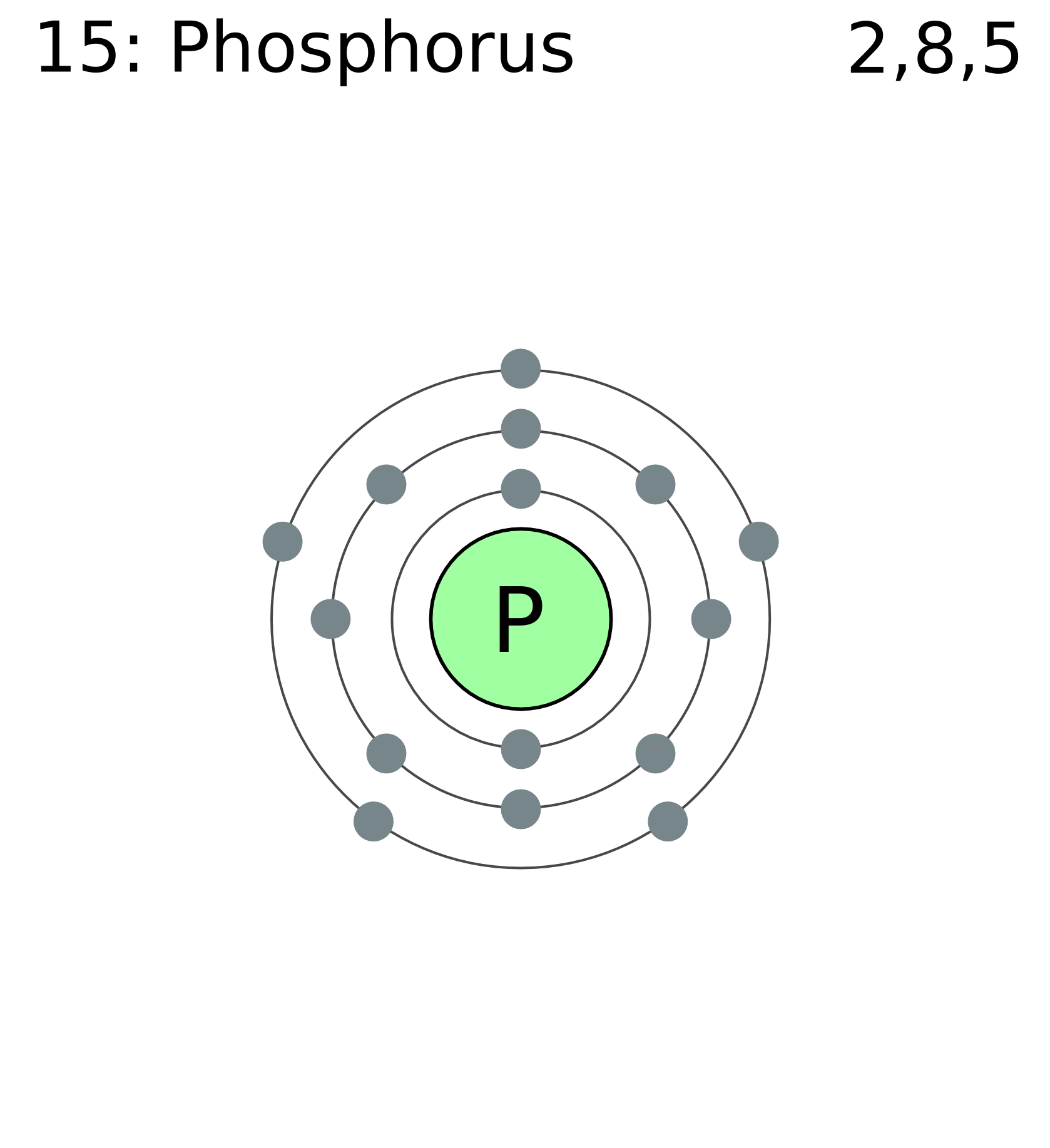
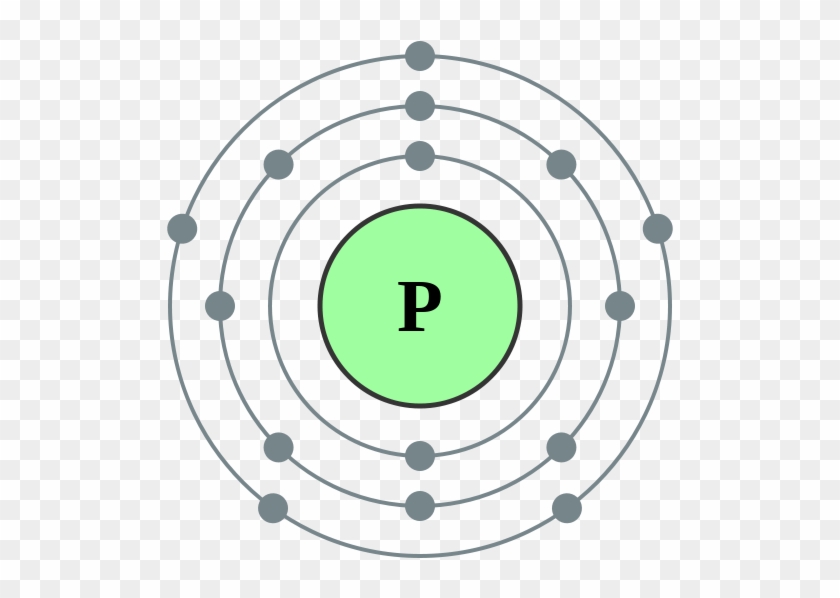
Located in the iii period. Web electron configuration of phosphorus is [ne] 3s2 3p3. Web based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. It is known that elemental phosphorous occurs mostly in two forms, white and red phosphorous. Web the complete electron configuration of phosphorus is 1s2.
Phosphorus Electron Configuration (P) with Orbital Diagram
Expert solution step by step solved in 2 steps with 2 images This element was discovered in 1669 by hennig brand. In this video we find the electron configuration of. 1s 2 2s 2 2p 6 3s 2 3p 3. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the.
Periodic Table Phosphorus Electron Configuration Periodic Table Timeline
1s 2 2s 2 2p 3: Electron configuration of boron (b) [he] 2s 2 2p 1: Web phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. It is in period 3 and in group 5 of the periodic table. Web in any atom with two or more electrons, the repulsion between the electrons makes energies.
Bohr Model Of A Phosphorus Atom Electron Configuration Of Sodium
Expert solution step by step solved in 2 steps with 2 images In this video we find the electron configuration of. Web based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. Web phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3. Electron configuration.
Phosphorus Definition, Facts, Symbol, Discovery, Property, Uses
The distribution of electrons are as 2 electrons in 1s subshell, electrons in 2s subshell,6 electrons in 2p subshell, 2 electrons in 3s subshell and the remaining 3 electrons in 3p subshell. Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. 317.3 k (44.15.
Electron configurations
The commonly used long form of the periodic table is designed to emphasize electron configurations.since it is the outermost (valence) electrons which are primarily involved in chemical interactions between atoms, the last electron added to an atom in the building. This element was discovered in 1669 by hennig brand. ∼860 k (∼590 °c, ∼1090 °f) boiling point: 553.7 k (280.5.
Phosphorus Electron Configuration YouTube
That gives a total of 5 valence electrons. 2 electrons in the 3 s subshell and 3 electrons in the 3 p subshell. 1s 2 2s 2 2p 6 3s 2 3p 3. Web the complete electron configuration of phosphorus is 1s2 2s2 2p6 3s2 3p3.phosphorus have 5 valence electrons around the nucleus and the atomic number is 15. P.
Electron configuration example for phosphorus Science, Chemistry
Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. When we write the configuration we'll put all 15 electrons in orbitals around the nucleus of the phosphorus atom. Some are hard to memorise (or predict), so what is the electron configuration of an atom.
Electron Configuration Of Carbon (C) [He] 2S 2 2P 2:
Write the long form electron configuration for phosphorous. Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Group 15 elements are also known as pnictogens. 1s 2 2s 2 2p 2:
Web This Page Shows The Electron Configurations Of The Neutral Gaseous Atoms In Their Ground States.
← electronic configurations of elements. In the case of phosphorus the abbreviated electron configuration is [ne] 3s2 3p3. ∼860 k (∼590 °c, ∼1090 °f) boiling point: Web based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p.
553.7 K (280.5 °C, 536.9 °F) Sublimation Point:
For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Demonstrate an understanding of atomic structure and chemical bonding, and how they relate to the physical properties of ionic, molecular, covalent network, and metallic substances. That gives a total of 5 valence electrons. 317.3 k (44.15 °c, 111.5 °f) red:
1S 2 2S 2 2P 1:
Web in any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. It is known that elemental phosphorous occurs mostly in two forms, white and red phosphorous. This element was discovered in 1669 by hennig brand. Web phosphorus has electron configuration, 1s 2 2s 2 2p 6 3s 2 3p 3.