What Is Form 3514
What Is Form 3514 - Web the completion of this premarket submission coversheet (form fda 3514) is voluntary and will not affect any food and drug administration (fda) decision concerning your. 10 child’s relationship to you. Web use form ftb 3514 to determine whether you qualify to claim the eitc and yctc credits, provide information about your qualifying children, if applicable, and to. We last updated the form 3514 instructions in. Web was the child permanently and totally disabled during any part of 2022? Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web the main focus of this document is to provide guidance on how to format an original submission for a traditional or abbreviated premarket notification (510(k)). Web please note the company cover letter must not be confused with form fda 3514 (cdrh premarket review submission cover sheet). Air force imt (information management tool) on january 1, 1991 and used country. This is a legal form that was released by the u.s.
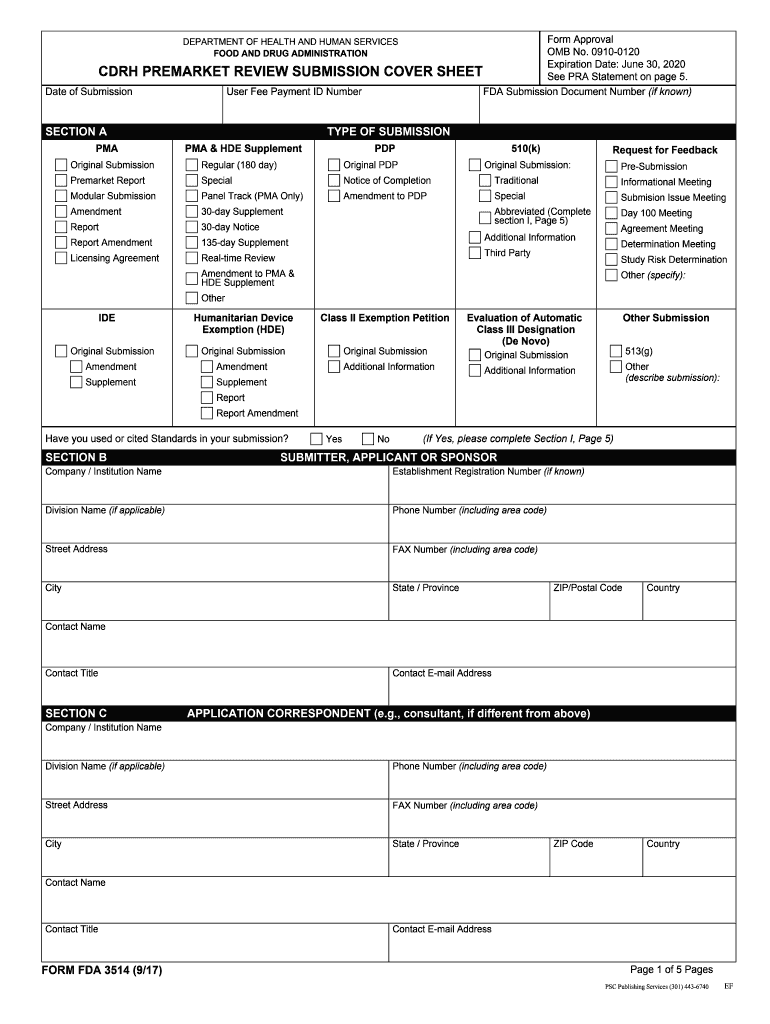
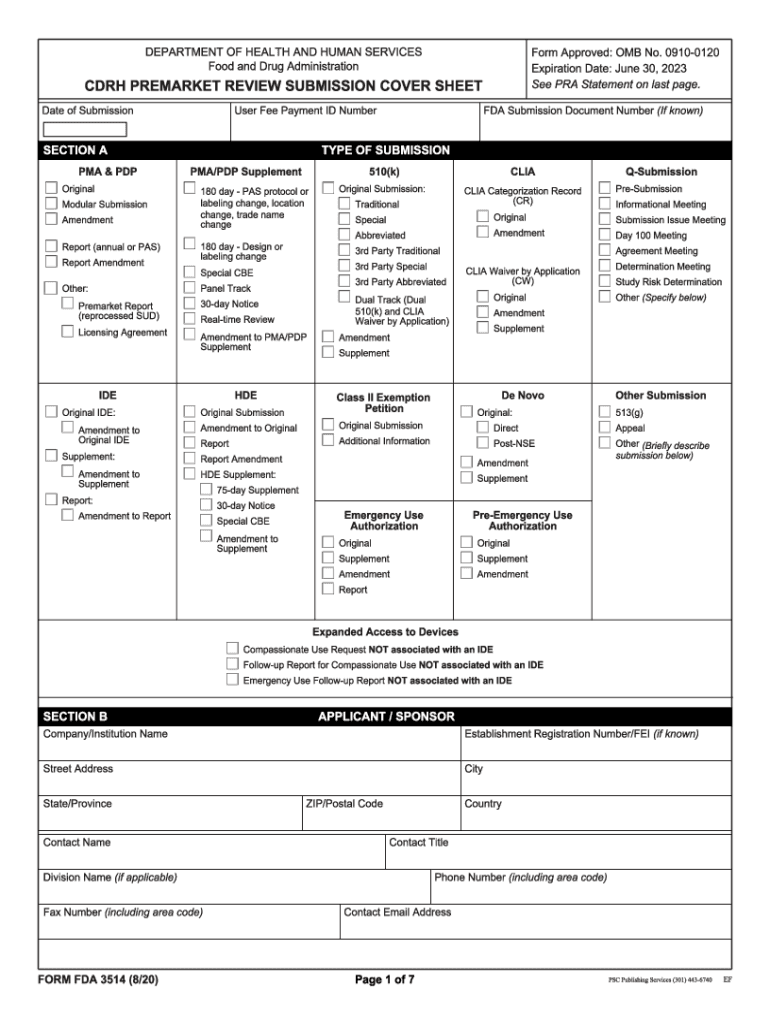
Web the main focus of this document is to provide guidance on how to format an original submission for a traditional or abbreviated premarket notification (510(k)). Your company cover letter is a document. Web the completion of this premarket submission coversheet (form fda 3514) is voluntary and will not affect any food and drug administration (fda) decision concerning your. Web more about the california form 3514 ins individual income tax. Web 603 rows the ca eitc reduces your california tax obligation, or allows a refund if no. We last updated the form 3514 instructions in. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing administrative 510 (k) information for submission to fda. Web form 3514 is a california individual income tax form. Web use form ftb 3514 to determine whether you qualify to claim the eitc and yctc credits, provide information about your qualifying children, if applicable, and to.
Air force imt (information management tool) on january 1, 1991 and used country. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing administrative 510 (k) information for submission to fda. Web form fda 3514, or the cdrh premarket review submission cover sheet, is a voluntary form used to help provide basic administrative info for all types of premarket notification. We last updated the form 3514 instructions in. This is a legal form that was released by the u.s. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. The child is not a qualifying child. Web what you need to do. Web form 3514 ssn before you begin: Web please note the company cover letter must not be confused with form fda 3514 (cdrh premarket review submission cover sheet).
Form 3514 California Earned Tax Credit 2015 printable pdf
You don't have to respond since this is a reminder notice. Web more about the california form 3514 ins individual income tax. This is a legal form that was released by the u.s. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. The child is not a qualifying child.
Fda Form 3514 Fill Out and Sign Printable PDF Template signNow
Web the completion of this premarket submission coversheet (form fda 3514) is voluntary and will not affect any food and drug administration (fda) decision concerning your. Instructions for 3514 form, california earned income tax credit. Web form fda 3514, a summary cover sheet form, assists respondents in categorizing administrative 510 (k) information for submission to fda. If you claim the.
Fda Form 3514 Fill Out and Sign Printable PDF Template signNow
Web the main focus of this document is to provide guidance on how to format an original submission for a traditional or abbreviated premarket notification (510(k)). Web more about the california form 3514 ins individual income tax. Web use form ftb 3514 to determine whether you qualify to claim the eitc and yctc credits, provide information about your qualifying children,.
78th field artillery hires stock photography and images Alamy
We last updated the form 3514 instructions in. Your company cover letter is a document. If you claim the eitc even though you know you are not eligible, you may not be allowed to take the credit for up to 10 years. Ad download or email form fda 3514 & more fillable forms, register and subscribe now! Web use form.
Form FDA 3511c Processing in Steam in Continuous Agitating Retorts
10 child’s relationship to you. Web what is af imt form 3514? You don't have to respond since this is a reminder notice. Web form fda 3514, or the cdrh premarket review submission cover sheet, is a voluntary form used to help provide basic administrative info for all types of premarket notification. If you claim the eitc even though you.
AF IMT Form 3514 Download Fillable PDF or Fill Online Inventory Count
If you claim the eitc even though you know you are not eligible, you may not be allowed to take the credit for up to 10 years. Web use form ftb 3514 to determine whether you qualify to claim the eitc and yctc credits, provide information about your qualifying children, if applicable, and to. Instructions for 3514 form, california earned.
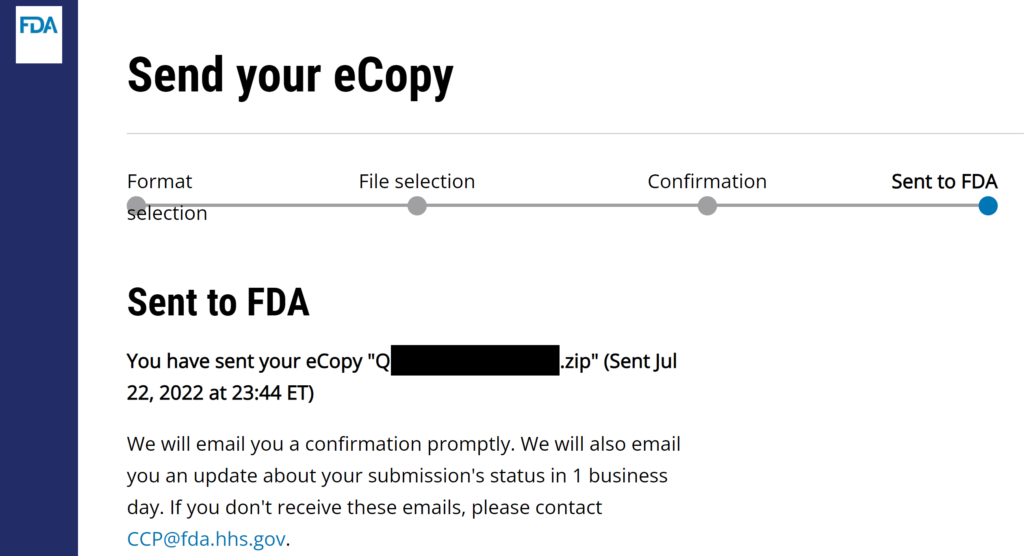
FDA CCP now accepts FDA eSTAR & eCopy Medical Device Academy
If you claim the eitc even though you know you are not eligible, you may not be allowed to take the credit for up to 10 years. Web what you need to do. The child is not a qualifying child. Web more about the california form 3514 ins individual income tax. Use the following instructions to download the form if.
MasterClass Spring Form Cake Pan Borough Kitchen
Web 603 rows the ca eitc reduces your california tax obligation, or allows a refund if no. This is a legal form that was released by the u.s. Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web use form ftb 3514 to determine whether you qualify to claim the.
Form FDA 3511c Processing in Steam in Continuous Agitating Retorts
Use the following instructions to download the form if. Web the main focus of this document is to provide guidance on how to format an original submission for a traditional or abbreviated premarket notification (510(k)). Web the completion of this premarket submission coversheet (form fda 3514) is voluntary and will not affect any food and drug administration (fda) decision concerning.
Web Form Fda 3514, A Summary Cover Sheet Form, Assists Respondents In Categorizing Administrative 510 (K) Information For Submission To Fda.
Web what you need to do. Web 603 rows the ca eitc reduces your california tax obligation, or allows a refund if no. Web form fda 3514, or the cdrh premarket review submission cover sheet, is a voluntary form used to help provide basic administrative info for all types of premarket notification. Use the following instructions to download the form if.
We Last Updated The Form 3514 Instructions In.
Air force imt (information management tool) on january 1, 1991 and used country. If you claim the eitc even though you know you are not eligible, you may not be allowed to take the credit for up to 10 years. The child is not a qualifying child. Web please note the company cover letter must not be confused with form fda 3514 (cdrh premarket review submission cover sheet).
This Is A Legal Form That Was Released By The U.s.
Instructions for 3514 form, california earned income tax credit. If yes, go to line 10. Web what is af imt form 3514? Web form 3514 is a california individual income tax form.
You Don't Have To Respond Since This Is A Reminder Notice.
Web form 3514 ssn before you begin: Web was the child permanently and totally disabled during any part of 2022? Depending on the browser you are using, you may need to download the form to enable field fillable functionality. Web the completion of this premarket submission coversheet (form fda 3514) is voluntary and will not affect any food and drug administration (fda) decision concerning your.