Which Of The Following May Form Linear Polymers
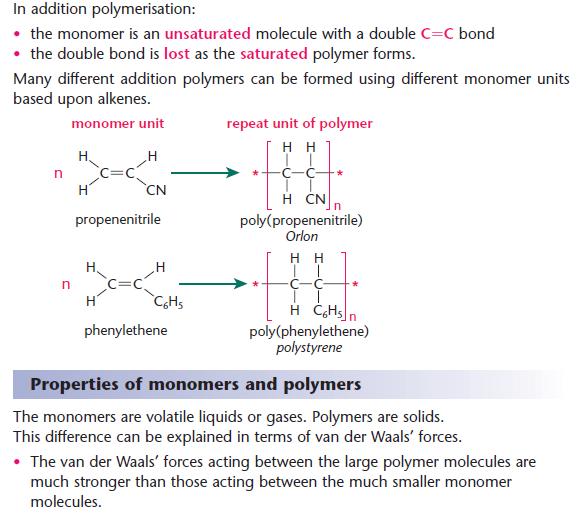
Which Of The Following May Form Linear Polymers - Web depending on the structure of the monomer and on the polymerization method employed, polymer chains may show different architectures. The monomers of polyvinyls have monomers as shown below. Web various polymer structures can be produced depending on the monomers and reaction conditions: The monomers in these are linked together to form a long chain. Web a linear polymer characterized by a repetition of ester groups along the backbone chain is called a polyester. These polymers are similar in structure to a long straight chain which identical links connected to each other. Web which type (s) of bond (s) is (are) found between atoms within hydrocarbon molecules? Note that for commercial synthesis the carboxylic acid components may actually be employed in the form of derivatives such as simple esters. Linear, branched, and network polymers.a polymer with a simple linear structure is formed. Web the following examples of condensation polymers are illustrative.
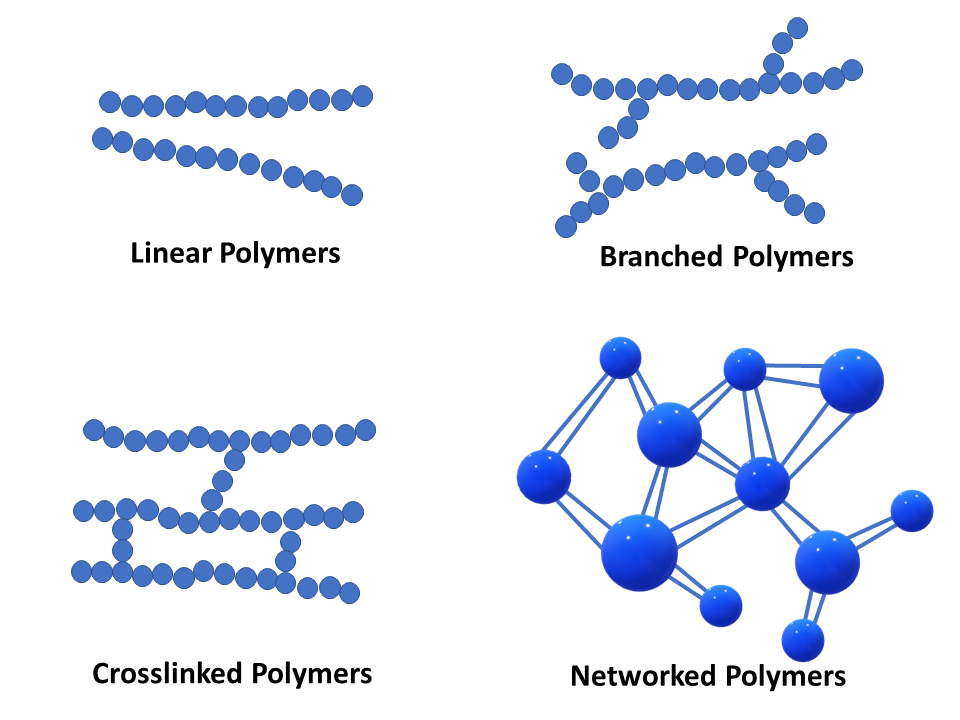
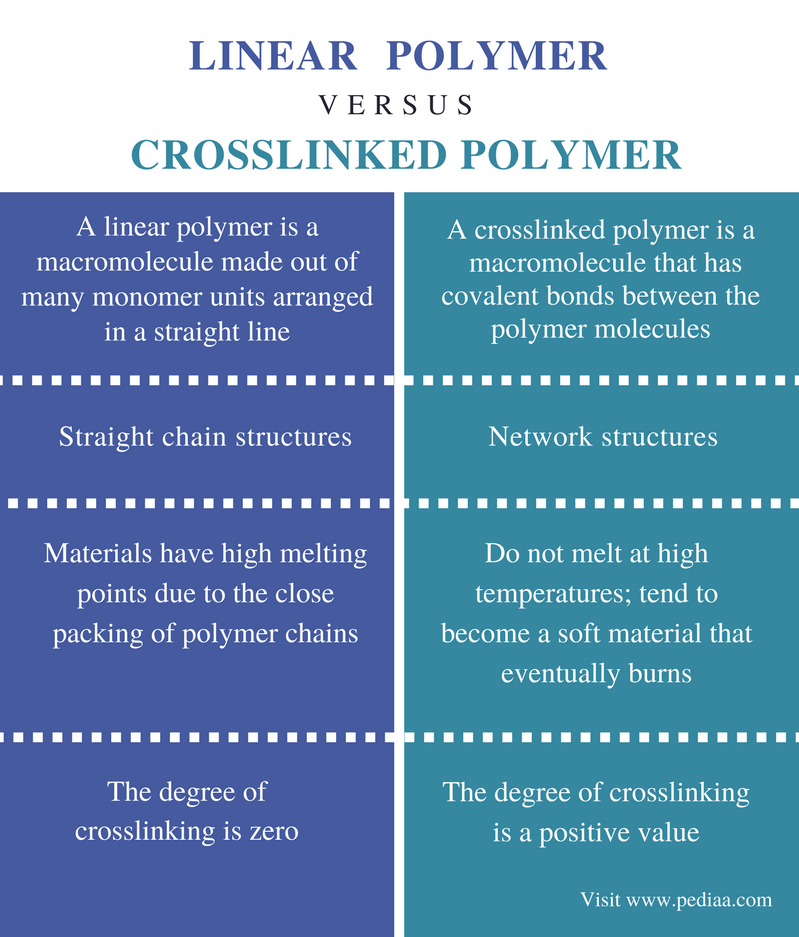
These polymers possess a single linear chain with zero branches. Starches, an important source of food energy. Web solution verified by toppr correct option is c) cellulose is a natural linear polymer, a long chain made by the linking of smaller glucose molecules. Linear, branched, and network polymers.a polymer with a simple linear structure is formed. A linear polymer may be considered to be a collection of linear chainlike molecules, such as shown in figure 1.40. Note that for commercial synthesis the carboxylic acid components may actually be employed in the form of derivatives such as simple esters. These polymers are composed of the main chain with more than one side chain and thus form branches. Cellulose and natural rubber are an example of. Also, the polymerization reactions for nylon 6 and. Those with high molecular weights (10,000 to 15,000 molecules) are employed in the.
In a solid plastic, a collection of these linear chains may be arranged in a completely random manner—looped, coiled, and. Web various polymer structures can be produced depending on the monomers and reaction conditions: A polymer may consist of linear macromolecules containing each only one unbranched chain. Cellulose and natural rubber are an example of. Which of the following may form linear polymers? These polymers have high melting points and are of higher density. These polymers are similar in structure to a long straight chain which identical links connected to each other. Which of the following form network polymers? These polymers are composed of the main chain with more than one side chain and thus form branches. Those with high molecular weights (10,000 to 15,000 molecules) are employed in the.
DrakeOwFernandez
The monomers of polyvinyls have monomers as shown below. How do the densities of crystalline and amorphous polymers of the same material that have identical molecular weights. These polymers are similar in structure to a long straight chain which identical links connected to each other. Which of the following form network polymers? Which of the following may form linear polymers?
Which of the following is a linear polymer ? YouTube
Long chains of 10,000 or more monomers can be packed closely to form a. Also, the polymerization reactions for nylon 6 and. Web polyvinyls and polyesters are commercial polymers with such linear backbones. Web a linear polymer characterized by a repetition of ester groups along the backbone chain is called a polyester. These polymers have high melting points and are.
(PDF) Latest Advances on the Synthesis of Linear ABCType Triblock
These polymers have high melting points and are of higher density. To account for the physical differences between the different types of polymers, the nature of the aggregate macromolecular structure, or morphology, of each substance must be considered.because polymer molecules are so. Starches, an important source of food energy. The monomers in these are linked together to form a long.
PPT Which of the following compounds may be polymers? PowerPoint
Web various polymer structures can be produced depending on the monomers and reaction conditions: A linear polymer may be considered to be a collection of linear chainlike molecules, such as shown in figure 1.40. Those with high molecular weights (10,000 to 15,000 molecules) are employed in the. These polymers possess a single linear chain with zero branches. In a solid.
Linear, Branched and Cross Linked Polymers and Polymer Crystallinity
Web which type (s) of bond (s) is (are) found between atoms within hydrocarbon molecules? If the segments are connected through the carbon atoms, then a linear polymer chain results. These polymers possess a single linear chain with zero branches. Web the following examples of condensation polymers are illustrative. Which of the following may form linear polymers?
Stimuliresponsive polymer materials showing a variety of topological
The monomers of polyvinyls have monomers as shown below. Those with high molecular weights (10,000 to 15,000 molecules) are employed in the. A regular and symmetrical linear chain, a low degree of polymerization, strong intermolecular forces, small and regular pendant groups, a slow rate of cooling, and oriented molecules. These polymers are similar in structure to a long straight chain.
Polymers Chemistry ALevel Revision
These polymers are similar in structure to a long straight chain which identical links connected to each other. Which of the following may form linear polymers? In a solid plastic, a collection of these linear chains may be arranged in a completely random manner—looped, coiled, and. These polymers possess a single linear chain with zero branches. Linear, branched, and network.
Solved Which of the following may form linear polymers?
If the segments are connected through the carbon atoms, then a linear polymer chain results. These polymers have high melting points and are of higher density. A polymer may consist of linear macromolecules containing each only one unbranched chain. Linear, branched, and network polymers.a polymer with a simple linear structure is formed. Note that for commercial synthesis the carboxylic acid.
Difference Between Linear and Crosslinked Polymer Definition
Polyvinyl chloride, polystyrene and polyethylene are examples of the polyvinyl family. Starches, an important source of food energy. Web six factors favor a polymer with a high percent crystallinity: Web which type (s) of bond (s) is (are) found between atoms within hydrocarbon molecules? A polymer may consist of linear macromolecules containing each only one unbranched chain.
Polymer Chemistry 5 Types of Classification of Polymers
How do the densities of crystalline and amorphous polymers of the same material that have identical molecular weights. Starches, an important source of food energy. Web solution verified by toppr correct option is c) cellulose is a natural linear polymer, a long chain made by the linking of smaller glucose molecules. Cellulose and natural rubber are an example of. Those.
Web Depending On The Structure Of The Monomer And On The Polymerization Method Employed, Polymer Chains May Show Different Architectures.
Web six factors favor a polymer with a high percent crystallinity: A linear polymer may be considered to be a collection of linear chainlike molecules, such as shown in figure 1.40. Web a linear polymer characterized by a repetition of ester groups along the backbone chain is called a polyester. Starches, an important source of food energy.
Web Polyvinyls And Polyesters Are Commercial Polymers With Such Linear Backbones.
Web solution verified by toppr correct option is c) cellulose is a natural linear polymer, a long chain made by the linking of smaller glucose molecules. The monomers of polyvinyls have monomers as shown below. Web which type (s) of bond (s) is (are) found between atoms within hydrocarbon molecules? The monomers in these are linked together to form a long chain.
Note That For Commercial Synthesis The Carboxylic Acid Components May Actually Be Employed In The Form Of Derivatives Such As Simple Esters.
These polymers are composed of the main chain with more than one side chain and thus form branches. To account for the physical differences between the different types of polymers, the nature of the aggregate macromolecular structure, or morphology, of each substance must be considered.because polymer molecules are so. These polymers have high melting points and are of higher density. Linear, branched, and network polymers.a polymer with a simple linear structure is formed.
In Some Polymers Shorter Chains Grow Off The Long Chain At Certain Intervals, So That A Branched Structure Is Formed.
Which of the following form network polymers? In a solid plastic, a collection of these linear chains may be arranged in a completely random manner—looped, coiled, and. A polymer may consist of linear macromolecules containing each only one unbranched chain. Web the following examples of condensation polymers are illustrative.