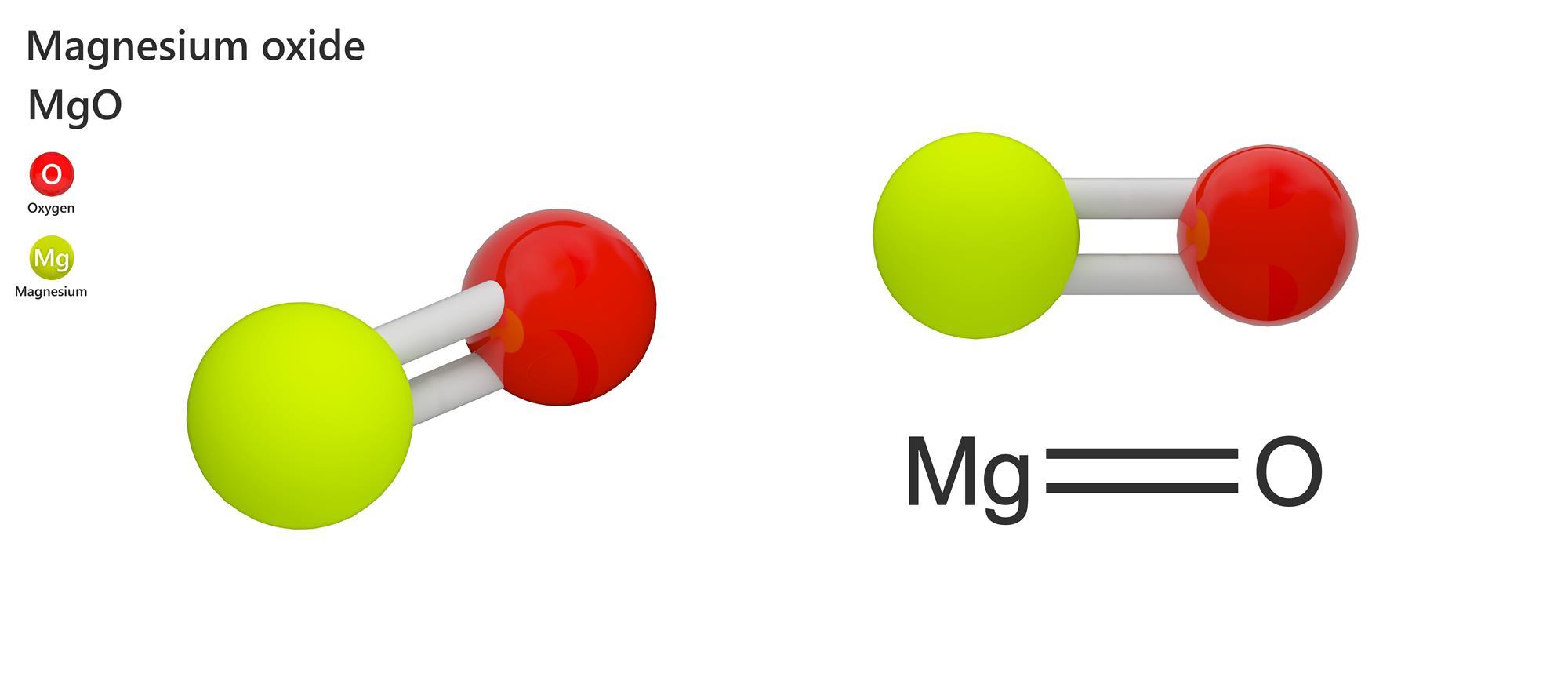Does Magnesium And Oxygen Form An Ionic Compound
Does Magnesium And Oxygen Form An Ionic Compound - Web why does magnesium and oxygen form an ionic bond? First examine the electron arrangement of the magnesium and oxygen atoms. Web does magnesium and oxygen form ionic compounds? Web is oxygen and magnesium and ionic compound? Web when magnesium and oxygen react and form am ionic compound, why is there only one oxygen atom for each magnesium atom instead of two? Web alternatively, use the crossing charges method shown in figure 3.3.2. Web since magnesium is a group 2a element, it forms +2 ions: Web may 27, 2023 by jay rana mgo (magnesium oxide) is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Web nomenclature, a collection of rules for naming things, is important in science and in many other situations.this module describes an approach that is used to name. See graphic on the left.
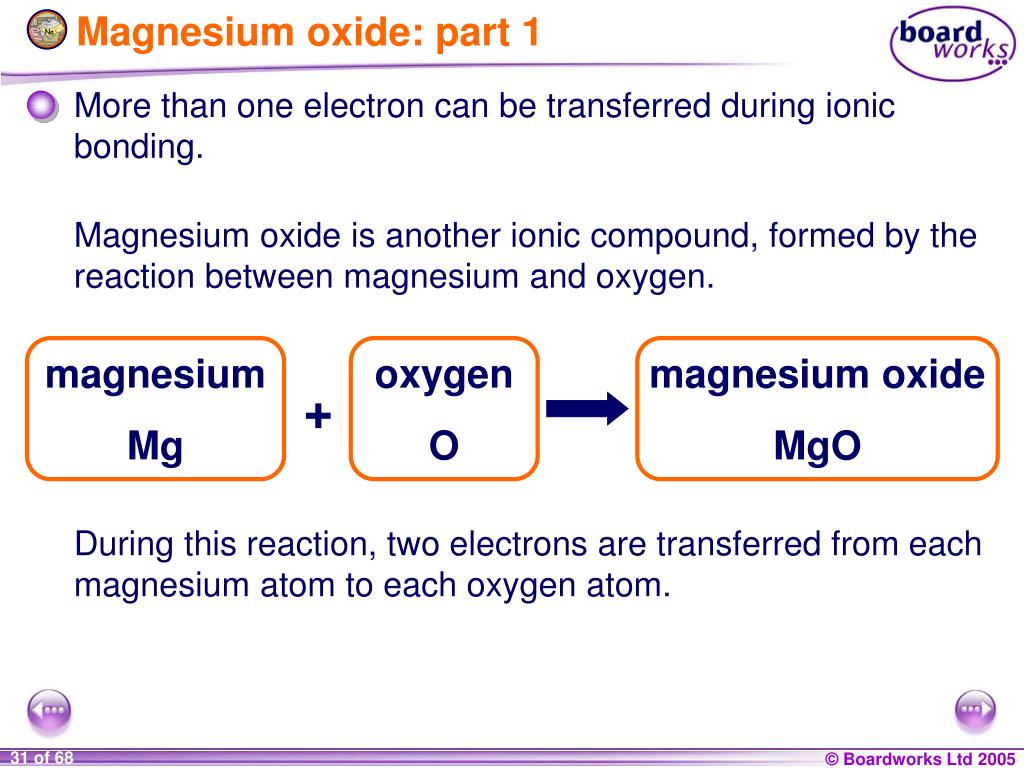
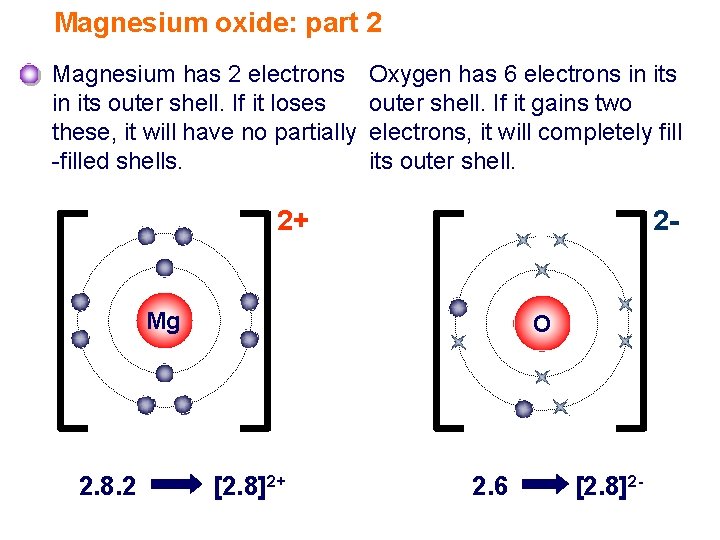
So based on these charges, we. Web does magnesium and oxygen form ionic compounds? Each magnesium atom transfers its two valence electrons to an oxygen atom to form the ionic. This problem has been solved!. First examine the electron arrangement of the magnesium and oxygen atoms. 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy in order for. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+. Web when magnesium and oxygen react and form am ionic compound, why is there only one oxygen atom for each magnesium atom instead of two? Web may 27, 2023 by jay rana mgo (magnesium oxide) is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Web magnesium forms magnesium oxide when it reacts with oxygen.
Magnesium and chlorine to find : Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Oxygen atoms have 6 electrons in their highest energy level, and require a further 2 to. Web may 27, 2023 by jay rana mgo (magnesium oxide) is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Web the ionic bond between magnesium and oxygen is stronger than the ionic bond between sodium and chlorine because of the greater charge on the ions. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+. After it burns, it forms a white powder of the magnesium oxide. Write the chemical formula for an ionic compound composed of each. 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy in order for. Web does magnesium and oxygen form ionic compounds?
Solved Which Of The Following Pairs Of Elements Are Likel...
So based on these charges, we. Natural abundance (%) half life. See graphic on the left. Oxygen atoms have 6 electrons in their highest energy level, and require a further 2 to. The reaction process occurs as two electrons move over from magnesium atoms to oxygen atoms.
Magnesium And Oxygen Ionic Compound slidesharetrick
When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+. Each magnesium atom transfers its two valence electrons to an oxygen atom to form the ionic. Web why does magnesium and oxygen form an ionic bond? Chemical formula for ionic compound q: 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best.
Media Portfolio
Each magnesium atom transfers its two valence electrons to an oxygen atom to form the ionic. So based on these charges, we. Chemical formula for ionic compound q: Oxygen atoms have 6 electrons in their highest energy level, and require a further 2 to. Web nomenclature, a collection of rules for naming things, is important in science and in many.
Ionic Bonding Elements are the simplest substances There
Web may 27, 2023 by jay rana mgo (magnesium oxide) is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. This problem has been solved!. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+. Web the two electrons donated from one magnesium atom are taken up.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web magnesium forms magnesium oxide when it reacts with oxygen. Oxygen atoms have 6 electrons in their highest energy level, and require a further 2 to. Web why does magnesium and oxygen form an ionic bond? See graphic on the left. Web does mangnesium and oxygen form an ionic bond?
Pin on Chemaddicts board
Web may 27, 2023 by jay rana mgo (magnesium oxide) is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Web the ionic bond between magnesium and oxygen is stronger than the ionic bond between sodium and chlorine because of the greater charge on the ions. Predict the chemical formula for the ionic.
Magnesium And Oxygen Ionic Compound slidesharetrick
First examine the electron arrangement of the magnesium and oxygen atoms. Web oxidation states and isotopes. Web magnesium forms magnesium oxide when it reacts with oxygen. Predict the chemical formula for the ionic compound formed by the elements al and l a:. Chemical formula for ionic compound q:
Magnesium Oxide Ionic Compound Diagram Diagram Media
Web when magnesium and oxygen react and form am ionic compound, why is there only one oxygen atom for each magnesium atom instead of two? Web does magnesium and oxygen form ionic compounds? Web oxygen and magnesium combine in a chemical reaction to form this compound. Each magnesium atom transfers its two valence electrons to an oxygen atom to form.
Magnesium Oxide Uses, Benefits, Dosage, Side Effects
Web the ionic bond between magnesium and oxygen is stronger than the ionic bond between sodium and chlorine because of the greater charge on the ions. Natural abundance (%) half life. See graphic on the left. Chemical formula for ionic compound q: Web oxygen and magnesium combine in a chemical reaction to form this compound.
magnesium reacts with oxygen to form magnesium oxide equation
Web does magnesium and oxygen form ionic compounds? This problem has been solved!. When an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+. Web oxidation states and isotopes. Web since magnesium is a group 2a element, it forms +2 ions:
Oxygen Atoms Have 6 Electrons In Their Highest Energy Level, And Require A Further 2 To.
Chemical formula for ionic compound q: Web the two electrons donated from one magnesium atom are taken up by an oxygen atom. First examine the electron arrangement of the magnesium and oxygen atoms. Write the chemical formula for an ionic compound composed of each.
Web Is Oxygen And Magnesium And Ionic Compound?
Magnesium and chlorine to find : Web does mangnesium and oxygen form an ionic bond? Web the ionic bond between magnesium and oxygen is stronger than the ionic bond between sodium and chlorine because of the greater charge on the ions. 4/28/2022 wiki user ∙ 14y ago study now see answer (1) best answer copy in order for.
Web Does Magnesium And Oxygen Form Ionic Compounds?
Web alternatively, use the crossing charges method shown in figure 3.3.2. See graphic on the left. Web may 27, 2023 by jay rana mgo (magnesium oxide) is an ionic compound because when the metal combines with nonmetal, it usually forms an ionic compound. Web when magnesium and oxygen react and form am ionic compound, why is there only one oxygen atom for each magnesium atom instead of two?
Predict The Chemical Formula For The Ionic Compound Formed By The Elements Al And L A:.
Each magnesium atom transfers its two valence electrons to an oxygen atom to form the ionic. Web when an ionic compound is formed from magnesium and oxygen, the magnesium ion has a 2+ charge, and the oxygen atom has a 2− charge. Web oxidation states and isotopes. Web oxygen and magnesium combine in a chemical reaction to form this compound.

.PNG)