Which Group Tends To Form 1 Ions
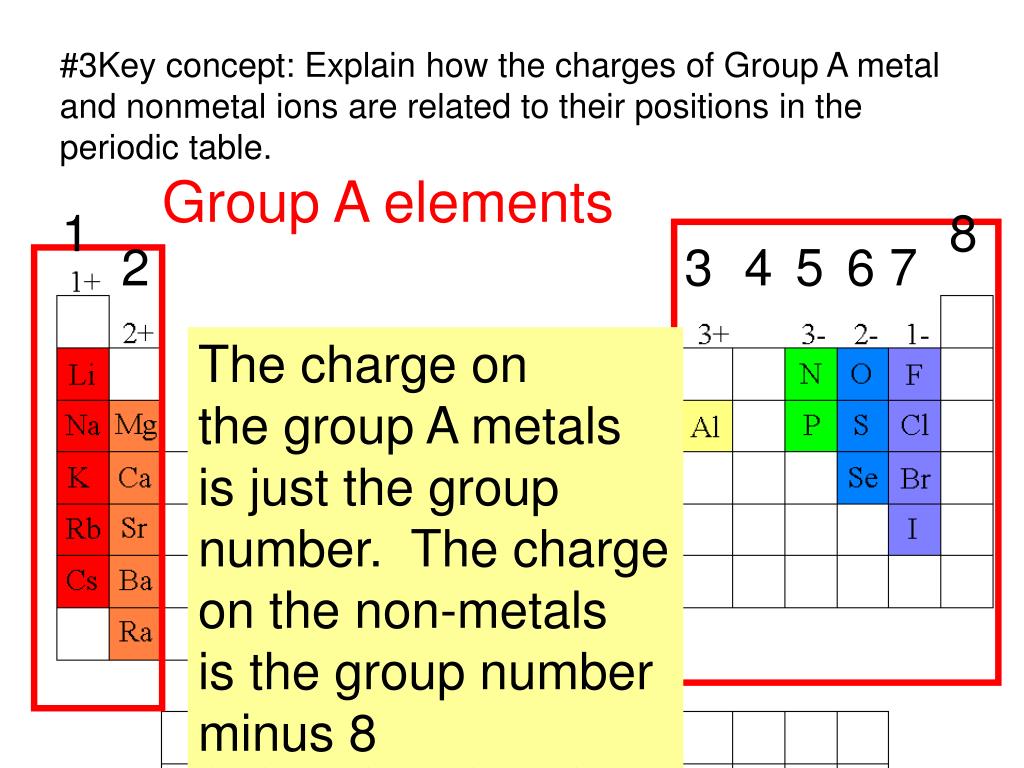
Which Group Tends To Form 1 Ions - Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions. The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. For example ,lets take sodium (na. Web group ia elements form ions with a +1 charge. Web combined science bonding, structure and the properties of matter revise video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. They then have the same number of electrons as the nearest noble gas:
For example ,lets take sodium (na. Web atoms of group 17 gain one electron and form anions with a 1− charge; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions. The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. They then have the same number of electrons as the nearest noble gas: The atoms of the elements toward the right end of the periodic table tend to undergo reactions such that they gain (or share) enough electrons to complete their. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. Group 2 elements form 2+ ions, and so on.
Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions. Rubidium (rb), cesium (cs), and francium (fr). The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. The atoms of the elements toward the right end of the periodic table tend to undergo reactions such that they gain (or share) enough electrons to complete their. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. They then have the same number of electrons as the nearest noble gas: The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Group 2 elements form 2+ ions, and so on. Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1:
Solved GENERAL QUESTIONS 13. Which group tends to form +1
Web combined science bonding, structure and the properties of matter revise video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions. Rubidium (rb), cesium (cs), and francium (fr). Web potassium,.
Ionic Compound Nomenclature Presentation Chemistry
The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration. For example as shown in figure 3.3, when a sodium (na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (na + ) with the electron configuration that looks like the. Group 2 elements form.
4.1f Predicting the ions formed by common main group elements YouTube
That is, group 1 elements form 1+ ions; Web atoms of group 17 gain one electron and form anions with a 1− charge; Web group ia elements form ions with a +1 charge. Web ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. Group 1 metals, the alkali metals, have the 1.
Naming Simple Ionic Compounds Pathways to Chemistry
Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. Web the 1st group (alkali metals) tends to form +1 ions. For example as shown in figure 3.3, when a sodium (na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (na + ) with the electron configuration.
table of elements chart
That is, group 1 elements form 1+ ions; The general outer electronic configuration of alkali metals are ns1,np0 if they lose one electron,it will attain stable electronic configuration. For example ,lets take sodium (na. The atoms of the elements toward the right end of the periodic table tend to undergo reactions such that they gain (or share) enough electrons to.
Periodic Table Groups 1 8 Names Periodic Table Timeline
For example ,lets take sodium (na. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized. Web group.
For Your Chemists Compound Interest
Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. The halogens, group 17,.
Ionic Compound Nomenclature Presentation Chemistry
Group 2 metals, the alkaline earth metals, have 2 valence electrons, and thus form m 2+ ions. For example ,lets take sodium (na. The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36.
Chem Ions Scientific Tutor
Web consistent with a tendency to have the same number of electrons as the nearest noble gas, when forming ions, elements in groups 1, 2, and 3 tend to lose one, two, and three electrons, respectively, to form cations, such as na + and mg 2+. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain.
Molecular and Ionic Compounds Introductory Chemistry Lecture & Lab
For example ,lets take sodium (na. Web atoms of group 17 gain one electron and form anions with a 1− charge; For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. They then have the same number of electrons as the nearest noble gas: The halogens, group 17,.
Web Group Ia Elements Form Ions With A +1 Charge.
Web atoms of group 17 gain one electron and form anions with a 1− charge; Rubidium (rb), cesium (cs), and francium (fr). For example as shown in figure 3.3, when a sodium (na) atom is ionized, it loses one of its 11 electrons, becoming a sodium ion (na + ) with the electron configuration that looks like the. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when oxidized.
Web Consistent With A Tendency To Have The Same Number Of Electrons As The Nearest Noble Gas, When Forming Ions, Elements In Groups 1, 2, And 3 Tend To Lose One, Two, And Three Electrons, Respectively, To Form Cations, Such As Na + And Mg 2+.
They lose one electron upon ionization, moving into the electron configuration of the previous noble gas. Group 2 elements form 2+ ions, and so on. Web atoms of group 17 gain one electron and form anions with a 1− charge; Web the 1st group (alkali metals) tends to form +1 ions.
The General Outer Electronic Configuration Of Alkali Metals Are Ns1,Np0 If They Lose One Electron,It Will Attain Stable Electronic Configuration.
The atoms of the elements toward the right end of the periodic table tend to undergo reactions such that they gain (or share) enough electrons to complete their. Atoms of group 16 gain two electrons and form ions with a 2− charge, and so on. They then have the same number of electrons as the nearest noble gas: The halogens, group 17, reach a full valence shell upon reduction, and thus form x− ions.
Atoms Of Group 16 Gain Two Electrons And Form Ions With A 2− Charge, And So On.
Web ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. That is, group 1 elements form 1+ ions; For example ,lets take sodium (na. Web combined science bonding, structure and the properties of matter revise video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge.

.PNG)


.PNG)

